Abstract
Translation of neuroprotective treatment effects from experimental animal models to patients with cerebral ischemia has been challenging. Since pathophysiological processes may vary across species, an experimental model to clarify human-specific neuronal pathomechanisms may help. We conducted a scoping review of the literature on human neuronal in vitro models that have been used to study neuronal responses to ischemia or hypoxia, the parts of the pathophysiological cascade that have been investigated in those models, and evidence on effects of interventions. We included 147 studies on four different human neuronal models. The majority of the studies (132/147) was conducted in SH-SY5Y cells, which is a cancerous cell line derived from a single neuroblastoma patient. Of these, 119/132 used undifferentiated SH-SY5Y cells, that lack many neuronal characteristics. Two studies used healthy human induced pluripotent stem cell derived neuronal networks. Most studies used microscopic measures and established hypoxia induced cell death, oxidative stress, or inflammation. Only one study investigated the effect of hypoxia on neuronal network functionality using micro-electrode arrays. Treatment targets included oxidative stress, inflammation, cell death, and neuronal network stimulation. We discuss (dis)advantages of the various model systems and propose future perspectives for research into human neuronal responses to ischemia or hypoxia.
Graphical Abstract

Similar content being viewed by others
Introduction
It has been challenging to translate neuroprotective treatment effects from experimental animal models to patients with cerebral ischemia. While numerous rodent studies have shown efficacy of divergent “neuroprotective” treatment strategies, in clinical trials these treatments have failed to improve functional outcomes (Ginsberg 2008). Failure of treatments in clinical trials have been discussed extensively, with possible explanations ranging from methodological weaknesses of animal studies to differences in treatment protocols and from infarct sizes to patient selection (Ginsberg 2008). However, genetic differences between rodents and humans that may translate to neurophysiological differences are relatively undiscussed. While rodents and humans share part of their DNA, various genes and proteins are expressed differently (Mestas and Hughes 2004). Therefore, cellular responses to cerebral ischemia or hypoxia may differ between rodents and humans. Furthermore, mechanisms of action or pathways that are targeted with neuroprotective treatments can vary (Ginsberg 2008). Consequently, rodent models might not be the optimal starting point to investigate the effect of cerebral ischemia on neuronal functionality and treatment strategies.
Various experimental studies addressed responses to ischemia or hypoxia in human-derived cell models. These consist of non-neuronal and neuronal models. The most used human in vitro model to study neuronal responses to ischemia or hypoxia is the neuroblastoma-derived SH-SY5Y cell model (Liu et al. 2018). This cell model is based on a cancerous cell line with the corresponding genetic characteristics, which may affect responses to ischemia or hypoxia and treatment strategies (Biedler et al. 1973, 1978). SH-SY5Y cells can, in principle, be differentiated into neuron-like cells upon stimulation (Shipley et al. 2016). However, the majority of research with SH-SY5Y cell models is conducted in undifferentiated SH-SY5Y cells. Only a small proportion of the research made use of protocols to differentiate SH-SY5Y cells into neuron-like cells. Furthermore, most studies focused on cell viability, neglecting neuronal functionality (Liu et al. 2018).
The recent advancement of human induced pluripotent stem cell (hiPSC) technology has created new opportunities to establish human neuronal in vitro models and investigate responses to ischemia or hypoxia. HiPSCs can be derived from healthy donors or patients, capturing person-specific genetic characteristics, and differentiated into neurons to generate neuronal networks (Frega et al. 2017; Mossink et al. 2021a). This allows investigation of the effect of, for example, a genetic mutation on neuronal functionality (Mossink et al. 2021b).
With this scoping review of the literature, we aim to provide an overview of (1) human neuronal in vitro models that have been used to study neuronal responses to ischemia or hypoxia, including characteristics of the various models, (2) parts of the ischemic pathophysiological cascade that have been investigated and (3) treatment targets that have been established in those human neuronal models. We will use the results as a starting point to discuss advantages and disadvantages of the various model systems, highlight current knowledge gaps, and propose possible future perspectives for research into human neuronal responses to ischemia or hypoxia.
Methods
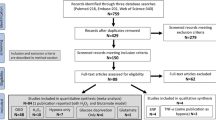
For this scoping review, we followed the PRISMA guidelines with regard to literature search, data collection, presentation of study characteristics and results, and discussion. However, we did not include a quality appraisal or risk of bias analysis.
To review investigated human neuronal in vitro models of cerebral ischemia or hypoxia, we applied a search in PubMed and SCOPUS databases until November 2021. We conducted the literature research with several combinations of key words and MeSH terms. We searched the literature with general terms for “human neuronal in vitro models” and the following specific search terms: “NT2-N”, “SK-N-SH”, “SH-SY5Y” or “hiPSC-derived neurons” (Liu et al. 2018). For selection of disease we used the MeSH terms “ischemic stroke” or “cerebral ischemia” and search terms “hypoxia” or “oxygen–glucose deprivation (OGD)” in combination with the different human neuronal in vitro models. One reviewer (EV) screened articles for eligibility based on the abstracts and methods. Review articles were used to screen reference lists. We only included studies with modeling of cerebral ischemia by oxygen–glucose deprivation or hypoxia. Studies were excluded when chemical simulation of ischemia was investigated. Flow chart is provided in the supplementary materials. For analyses of effects of potential neuroprotective treatments, we included studies on treatments that were applied during or after ischemia/hypoxia, and excluded studies that investigated the effect of pre-treatment (i.e. a treatment started before initiation of hypoxia or ischemia). We extracted the cell type, the level of differentiation towards neurons (if applicable), the way in which ischemia or hypoxia was modelled (including duration and recovery period), treatment strategy (if applicable), outcome measures, and results from the included studies. All results of the scoping review are presented in a descriptive way.
Results
We included 147 papers on four different human neuronal in vitro models of cerebral ischemia/hypoxia.
Model Characteristics
Model characteristics are summarized Fig. 1 and Table 1. Of the 147 included papers, 145 were on cell models derived from unhealthy donors (neuroblastoma SK-N-SH (n = 11) and SH-SY5Y (n = 132)) or carcinoma (differentiated NT2-N (n = 2)). SK-N-SH is a cell model directly derived from a neuroblastoma cell line. SH-SY5Y cell model is based on a subclone derived from the SK-N-SH cell line. These can both be differentiated into neuron-like cells by stimulation with retinoic acid (RA). However, the majority of studies on neuronal response to ischemia/hypoxia is conducted in undifferentiated cell models (SK-N-SH undifferentiated n = 9 and differentiated n = 2; SH-SY5Y undifferentiated n = 119, and differentiated n = 13). Two studies were conducted on neurons differentiated from hiPSCs of healthy donors (Juntunen et al. 2020; Pires Monteiro et al. 2021). Examples of cultured undifferentiated SH-SY5Y and hiPSC-derived neuronal networks are presented in Fig. 2, showing that undifferentiated SH-SY5Y cells have short truncated processes and do not express the neuronal markers microtubule-associated protein 2 (MAP2) and Synapsin 1/2 positive synaptic puncta. While hiPSC-derived neuronal networks show long neurites and express MAP2 and Synapsin 1/2 positive synaptic puncta.
Pie chart representing the distribution of human neuronal in vitro models used in experimental cerebral ischemia studies. The majority of the research is conducted in SH-SY5Y cell models. hiPSCs human induced pluripotent stem cells. Differentiated NT2-N = teratocarcinoma-derived Ntera2/D1 neuron-like cells
Representative images of undifferentiated SH-SY5Y cells and hiPSC-derived neuronal networks. Bright field images of undifferentiated SH-SY5Y cells (A) and hiPSC-derived neuronal networks (B) (× 4 magnification) (scalebar = 100 μm). Immunofluorescent images of undifferentiated SH-SY5Y cells (C) and hiPSC-derived neuronal networks (D) (× 60 magnification) (scalebar = 30 μm). Undifferentiated SH-SY5Y cells have short truncated processes and do not express the neuronal markers MAP2 and Synapsin 1/2 positive puncta. HiPSC-derived neuronal networks have extended neurites and express neuronal markers MAP2 and Synapsin 1/2 positive puncta. Blue = DAPI (nuclei), Green = micro-tubule associated protein 2 (MAP2), Red = Synapsin 1/2
Cerebral ischemia was modeled by oxygen–glucose deprivation (OGD; n = 142) or hypoxia (n = 5). All studies used immunocytochemical techniques to investigate cell survival, protein expression linked with cell death or inflammation, or factors related to oxidative stress. Neuronal functionality was assessed in only one study in which electrophysiological measurements were performed with micro-electrode arrays (MEAs) (Pires Monteiro et al. 2021).
Effects of Ischemia or Hypoxia on Human Cell Models
The pathophysiological cascade that follows upon cerebral ischemia/hypoxia is extensive and complex. In SH-SY5Y, SK-N-SH, NT2-N and hiPSC-derived cell models, several steps of this pathophysiological cascade have been investigated. These steps are summarized in Table 2 and explained below.
SH-SY5Y and SK-N-SH Cell Models
Calcium Homeostasis Dysregulation
Ischemia induced membrane depolarization leads to opening of calcium channels, ultimately resulting in an increase in intracellular Ca2+. With insufficient ATP production, Ca2+ extruders (e.g. Na+/Ca2+ exchanger) stop working, causing a pathological increase of the intracellular and mitochondrial Ca2+ concentration. The Na+/Ca2+ exchanger 1 (NCX1) has been investigated in one study using undifferentiated SH-SY5Y cells. The results showed that NCX1 was repressed during OGD by the RE1-silencing transcription factor (REST) (Formisano et al. 2013). In undifferentiated SK-N-SH cells increased levels of intracellular calcium was found during OGD (n = 1) (Lehane et al. 2013).
Oxidative/Nitrosative Stress
Oxidative stress results from imbalance between pro- and anti-oxidants, that in turn results in excessive formation of reactive oxygen species (ROS). Various negative effects of ROS on DNA, proteins or lipids have been established in SH-SY5Y and SK-N-SH cell models of cerebral ischemia.
The first ROS that is produced during ischemia/hypoxia is superoxide anion (O2−), which is the precursor of most other ROS. One study in undifferentiated SH-SY5Y cells found an increase in O2− (Marmol et al. 2021). Superoxide dismutases (SODs) convert superoxide to hydrogen peroxide (H2O2), which can be removed by glutathione peroxidase (GPx). GPx was decreased after ischemia/hypoxia in undifferentiated SH-SY5Y and SK-N-SH cells (Wang et al. 2020a; Wang and Xu 2021). SODs prevent the formation of highly aggressive ROS. Ten studies in undifferentiated SH-SY5Y cells and one in undifferentiated SK-N-SH cells found decreased levels of SODs during ischemia/hypoxia (Di et al. 2021; Dong et al. 2021; Feng et al. 2021; Gao et al. 2017, 2020; Li et al. 2019b; Liu et al. 2019b; Wang et al. 2019b, 2020a; Wang and Xu 2021; Wen et al. 2020; Xu et al. 2020b).
Glutathione (GSH) is an antioxidant that can prevent damage to cellular components by ROS. The ratio of reduced GSH to oxidized glutathione (GSSG) is a measure of cellular oxidative stress. This ratio has been investigated in one study using undifferentiated SH-SY5Y cells (Guo et al. 2009). The results showed a decreased ratio by increased oxidized glutathione. This study also investigated the level of cellular nicotinamide adenine dinucleotide phosphate (NADPH) content. NADPH provides oxidation–reduction which protects against ROS allowing the regeneration of GSH. There was no difference in NADPH content between normoxia or hypoxia treated undifferentiated SH-SY5Y cells (Guo et al. 2009). NADPH oxidase was involved in post-ischemic cell death in differentiated SH-SY5Y cells (n = 1) (Beske and Jackson 2012). One other study investigated the level of GSH in undifferentiated SH-SY5Y cells and found that there was a decrease in GSH during hypoxia, leading to increased cellular oxidative stress (Wang et al. 2018a). Another anti-oxidant that has been investigated in undifferentiated SH-SY5Y cells is catalase. This enzyme was reduced after ischemia/hypoxia (Wang et al. 2019b).
Besides anti-oxidants, free radical scavengers were investigated in an undifferentiated SH-SY5Y cell model of cerebral ischemia, revealing that spermine, spermidine and other polyamines were elevated (Shin et al. 2016). ROS can also be decreased by hypoxia inducible factor 1 (HIF-1). In undifferentiated SH-SY5Y cells it was found that HIF-1 regulated redox status after hypoxic exposure (Guo et al. 2009).
Another factor that counteracts ROS formation is the activation of the redox sensitive transcription factor NF-E2 related factor 2 (NRF-2). This is a master regulator of enzymes involved in anti-oxidant glutathione synthesis and in elimination of ROS. Thus, NRF-2 plays a role in the defense against oxidative stress. NRF-2 and other cytoprotective enzymes have been investigated in seven studies using undifferentiated SH-SY5Y cells. The results showed increased expression of NRF-2 (Lin-Holderer et al. 2016; Liu et al. 2019b; Park et al. 2017; Ruan et al. 2020; Shi et al. 2019; Wang and Xu 2021; Zhi et al. 2020) and the cytoprotective enzymes NAD(P)H dehydrogenase [quinone] 1 (NQO1) and hemeoxygenase-1 (HO-1) (Liu et al. 2019b; Park et al. 2017; Ruan et al. 2020; Shi et al. 2019; Wang and Xu 2021; Zhi et al. 2020) after ischemia/hypoxia.
Malondialdehyde (MDA) is a marker of oxidative stress. It is a highly cytotoxic agent and has been investigated to assess oxidative stress in seven studies using undifferentiated SH-SY5Y cells and one study using undifferentiated SK-N-SH cells. MDA levels increased during hypoxia/ischemia, which suggested increased oxidative stress (Dong et al. 2021; Gao et al. 2017, 2020; Wang et al. 2018a, 2019b, 2020a; Wang and Xu 2021; Xu et al. 2020b). Nicotinamide adenine dinucleotide phosphate oxidase (NOX) is an important player in ROS generation. NOX expression was increased in undifferentiated SH-SY5Y cell models of cerebral ischemia (n = 3) (Hong et al. 2017; Hsieh et al. 2021; Wu et al. 2020a).
The neuronal NO synthase (nNOS) enzymes are expressed in neurons and contribute to nitrite oxide (NO) production in ischemic processes, leading to nitrosative stress. nNOS has been investigated in one study using undifferentiated SH-SY5Y cells and the results showed reduced expression of nNOS enzymes during ischemic stress (Tajes et al. 2013). Nitrosative stress is mediated by peroxynitrites, that are produced by NO. Peroxynitrites play an important role in the development of neuroinflammation. One study found increased peroxynitrite levels after hypoxia in undifferentiated SH-SY5Y cells (Marmol et al. 2021). The level of nitrite has been investigated in undifferentiated SH-SY5Y cells and the results showed increased intracellular nitrite concentration after ischemia/hypoxia (Hsieh et al. 2021).
ROS regulates cell ferroptosis by multiple signaling pathways like the Janus kinase and signal transducer and activator of transcription (JAK/STAT). JAK2 has been investigated in undifferentiated SH-SY5Y cells in OGD. The results showed increased expression of JAK2 and STAT3 (n = 2) (Huang et al. 2018; Xu et al. 2020b).
Oxidative stress can lead to endoplasmic reticulum (ER) stress-related protein expression. ER stress markers are inducers of neuronal cell death and increase the tissue injury after cerebral ischemia. In three studies with undifferentiated SH-SY5Y cells, several ER stress related proteins were increased, such as 78 kDa glucose-regulated protein/binding immunoglobulin protein (GRP78/BIP), phosphorylated inositol-requiring enzyme-1 alpha (p-IRE1α), X-box binding protein 1 (XBP1), C/EBP homologous protein (CHOP), and activating transcription factor 6 (ATF6) (Hong et al. 2017; Lu et al. 2019; Wang et al. 2018a). In two studies with differentiated SH-SY5Y cells, Endoplasmic Reticulum Metallopeptidase 1 (ERMP1), GRP78, phosphorylated protein kinase-like endoplasmic reticulum kinase (p-PERK), phosphorylated α-subunit of eukaryotic initiation factor 2 (p-EIF2α) and CHOP were increased (Chi et al. 2019; Pan et al. 2020).
The transforming growth factor (TGF)/SMAD pathway has been implicated in oxidative stress. TGF expression was increased in an undifferentiated SH-SY5Y cell model of cerebral ischemia, together with increased phosphorylation of SMAD2 and SMAD3. Ischemia/hypoxia promoted translocation of SMAD3 from the cytoplasm to the nucleus (Yang et al. 2021b).
Oxidative stress can cause DNA damage. A multifunctional protein that is involved in the base-excision repair of oxidative DNA damage is Apurinic/apyrimidinic endonuclease 1 (APE1). The expression level of APE1 decreased during ischemia/hypoxia in undifferentiated SH-SY5Y cells (n = 1) (Wu et al. 2020a).
Mitochondrial Dysfunction
Neuronal ischemia/hypoxia causes mitochondrial dysfunction along several ways: oxidative stress with mitochondrial DNA mutations, dysfunction of the mitochondrial respiratory chain, altered membrane permeability with Ca2+ accumulation, and disturbance of mitochondrial defense systems (Ham and Raju 2017).
One study in undifferentiated SH-SY5Y cells showed that the mitochondrial permeability transition pore is opened with OGD by translocation of P53 to mitochondria and activation of Cyclophilin D (Zhao et al. 2013). The consequent mitochondrial membrane depolarization has been found in undifferentiated SH-SY5Y cells (n = 3) (Agudo-Lopez et al. 2010; Park et al. 2017; Wang et al. 2018b). Mitochondrial membrane permeabilization by opening of the mitochondrial permeability transition pore and loss of the mitochondrial membrane potential (MMP) has also been established in three studies in undifferentiated and in two studies differentiated SH-SY5Y cells (Lin et al. 2020; Liu et al. 2019a; Park et al. 2017; Sriwastva et al. 2020; Wang et al. 2019b). Normal mitochondrial function was reduced in undifferentiated SH-SY5Y cells after OGD (Herrmann et al. 2013).
Nuclear respiratory factor-1 and mitochondrial transcription factor A were decreased in undifferentiated SH-SY5Y cells after ischemia/hypoxia (Lin et al. 2020). Cytochrome oxidase activity was decreased (Lin et al. 2020). When mitochondrial dysfunction occurs, cells usually remove the dysfunctional mitochondria with mitophagy. Ischemia/hypoxia induced mitophagy has been established in one study in undifferentiated SH-SY5Y cells (Ping et al. 2021).
Inflammation
Cerebral ischemic injury and reperfusion cause a detrimental inflammatory cascade. Cytokines play a crucial role. Several cytokines have been investigated in human neuronal in vitro models of cerebral ischemia. These cytokines can be divided in pro-inflammatory and anti-inflammatory cytokines. In undifferentiated SH-SY5Y (n = 12) and SK-N-SH (n = 1) cell models, expression of pro-inflammatory cytokines (interleukin-1β, interleukin-6, interleukin-18 and interferon γ) was increased after ischemia/hypoxia (Chai et al. 2020; Dong et al. 2021; Huang et al. 2021; Li et al. 2019a, b; Li and Ma 2020; Liu et al. 2021; Meng et al. 2021; Shi et al. 2020, 2021; Yin et al. 2021; Zhao and Wang 2020). Pro-inflammatory cytokines like IL-1β and IL-18 can be activated by NLR family pyrin domain containing 3 (NLRP3) inflammasomes, which comprises NLRP3, ASC, and pro-caspase-1. The formation of the NLRP3 inflammasome was increased in undifferentiated SH-SY5Y cells (n = 1), differentiated SH-SY5Y (n = 1) and undifferentiated SK-N-SH cells (Fu et al. 2020; Yin et al. 2021).
Anti-inflammatory cytokines can be induced by V-maf musculoaponeurotic fibrosarcoma oncogene homolog B. This transcriptional activator was found to be decreased after ischemia/hypoxia in one study using undifferentiated SH-SY5Y cells (Zhang et al. 2020a). The anti-inflammatory factor interleukin-10 was reduced after ischemia/hypoxia in one study with undifferentiated SH-SY5Y cells (Dong et al. 2021).
Tumor necrosis factor (TNF) is released by cells under stress and stimulates the immune response. The expression of TNF is only investigated in undifferentiated SH-SY5Y cells during and after ischemia/hypoxia (n = 15). The results show an increase in TNF expression induced by ischemia/hypoxia, which suggests increased inflammatory response (Chai et al. 2020; Dong et al. 2021; Hao et al. 2015; Huang et al. 2021; Landgraf et al. 2020; Li et al. 2019a; Li and Ma 2020; Liu et al. 2021; Meng et al. 2021; Shi et al. 2020, 2021; Yang et al. 2014; Zhao and Wang 2020; Zhi et al. 2020). Another protein that belongs to the TNF family is Fas ligand (FASL). When this protein binds to its receptor, it induces apoptosis. FASL has been investigated in two studies using undifferentiated SH-SY5Y cells and the results showed Fas/FASL interaction was increased. This was correlated with a higher apoptosis rate (Chen et al. 2015; Zhang et al. 2016b). Another study has found that the levels of TNF and interleukin-6 (IL-6) were regulated by F-Box Protein 3 (FBXO3). Ischemia/hypoxia induced increased levels of FBXO3, which was correlated with increased levels of TNF and IL-6 (Li et al. 2019b). Various pro-inflammatory factors (nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), Dual Specificity Phosphatase 14 (DUSP14), Toll-like receptor 4 (TLR4), Myeloid differentiation primary response 88 (MYD88), SIRTUIN 1 (SIRT1), SIRTUIN 6 (SIRT6), High mobility group box 1 (HMGB1), chemokine (C-X-C motif) ligand 1 (CXCL1)) were increased in 14 studies with undifferentiated SH-SY5Y cells (Chen et al. 2021a; Dong et al. 2021; Hao et al. 2015; Huang et al. 2021; Janssen et al. 2021; Jiang et al. 2012; Lee et al. 2013; Li et al. 2019b; Ruan et al. 2021; Shi et al. 2020, 2021; Tian et al. 2020; Yang et al. 2014; Zhao and Wang 2020; Zhi et al. 2020).
Inhibitor of κB (IκB) kinase is an enzyme involved in propagating the cellular response to inflammation. It activates NF-κB. This kinase is investigated in four studies using undifferentiated SH-SY5Y cell, two of which investigated IκB kinase in combination with the activity of NF-κB (Dong et al. 2021; Huang et al. 2021; Jiang et al. 2012; Li et al. 2019b). The results showed an increase in IκB kinase.
Autophagy
Intracellular autophagy is activated in different cell types of the brain upon nutrient starvation or metabolic stress. It is the phagocytosis of cytoplasmic materials, which are captured by autophagosomes and fuse with lysosomes to form autolysosomes and are then degraded. Several factors related to autophagy after ischemia/hypoxia have been investigated in human neuronal in vitro models.
Ischemia/hypoxia increased the level of microtubule-associated protein 1 light chain 3 (LC3), as was found in undifferentiated (n = 9) (Lai et al. 2020; Liu et al. 2016, 2021; Niu et al. 2018; Shi et al. 2012; Wang et al. 2017, 2019c; Zhi et al. 2020; Zhou et al. 2019) and differentiated SH-SY5Y cells (n = 2) (Cheng et al. 2019; Zhang et al. 2019b). Connexin32 (CX32) was found to inhibit the autophagic effect of nuclear receptor 4A1 (NUR77) during ischemia/hypoxia in one study using undifferentiated SH-SY5Y cells (Ping et al. 2021). Increased Beclin-1 expression was found to increase the level of autophagy in four studies using undifferentiated SH-SY5Y cells (Shi et al. 2012; Wang et al. 2017, 2019c; Zhi et al. 2020) and one study using differentiated SH-SY5Y cells during ischemia/hypoxia (Cheng et al. 2019).
Dual specificity protein phosphatase 5 (DUSP5) increased the level of autophagy through DUSP5-ERK1/2 axis according to one study in undifferentiated SH-SY5Y cells (Wang et al. 2017). Phosphorylation of mammalian target of rapamycin (mTOR) was found to be increased, which was correlated with increased level of autophagy in five studies using undifferentiated SH-SY5Y cells (Guo et al. 2018; Liu et al. 2021; Wang et al. 2019b; Zhi et al. 2020; Zhou et al. 2019) and one study using differentiated SH-SY5Y cells (Cheng et al. 2019).
Cell Death
Cell death is a much studied final common path in human neuronal in vitro models of cerebral ischemia. A total of 59 studies in undifferentiated (Agudo-Lopez et al. 2010; Castri et al. 2014; Chen et al. 2019; Dong et al. 2019, 2021; Du et al. 2012; Feng et al. 2021; Gao et al. 2017, 2020; He et al. 2014; Hu et al. 2020; Huang et al. 2018, 2021; Jia et al. 2021; Lee et al. 2013; Li and Ma 2020; Li et al. 2020a; Liu et al. 2019b; Lu et al. 2019; Luan et al. 2013; Marutani et al. 2012; Meng et al. 2021; Nampoothiri and Rajanikant 2019; Niu et al. 2018; Park et al. 2017; Ruan et al. 2020; Shan et al. 2022; Shi et al. 2021; Song et al. 2019; Tian et al. 2020; Wang et al. 2013, 2017, 2018b, c, 2019b, c, 2020b, 2021a, b; Wang and Xu 2021; Wen et al. 2020; Wu et al. 2020a, b; Xing et al. 2021; Xu et al. 2020a, b; Yan et al. 2015, 2020; Yang et al. 2014, 2021a; Yao et al. 2019; Yi et al. 2020; Zappala et al. 2021; Zhang et al. 2016a, b, 2019a, b, 2020; Zhao and Wang 2020; Zhao et al. 2013; Zhi et al. 2020; Zhou et al. 2019; Zuo et al. 2020) and four studies in differentiated SH-SY5Y cells (Chi et al. 2019; Sriwastva et al. 2020; Xu et al. 2020a; Zhang et al. 2019b) showed a dose- and time-dependent increase in cell death after hypoxia as measured with flow cytometry or immunofluorescence. Similarly, an increase in cell death after ischemia/hypoxia was measured in three studies with undifferentiated SK-N-SH (Jin et al. 2020; Li et al. 2017; Zhou et al. 2016) and in one study in differentiated SK-N-SH cells (Wang et al. 2020a).
In SH-SY5Y and SK-N-SH cell models, cell death rate after ischemia/hypoxia has been investigated extensively. Eighty-three papers found increased cell death rates after ischemia/hypoxia in undifferentiated SH-SY5Y cells (Castri et al. 2014; Chan et al. 2015; Chang et al. 2017; Chen 2004; Chen et al. 2019; Dong et al. 2019; Fang et al. 2020; Feng et al. 2021; Formisano et al. 2013; Gao et al. 2017, 2020; Guo et al. 2018, 2019; Hong et al. 2017; Hsieh et al. 2021; Hu et al. 2020; Huang et al. 2018, 2021; Janssen et al. 2021; Jia et al. 2021; Jimenez-Almarza et al. 2019; Karuppagounder et al. 2013; Landgraf et al. 2020; Li et al. 2019b, 2020b; Lin-Holderer et al. 2016; Liu 2016; Liu et al. 2016, 2018, 2019b; Lu et al. 2019; Luan et al. 2013; Marmol et al. 2021; Marutani et al. 2012; McCune et al. 2016; Meng et al. 2021; Nampoothiri and Rajanikant 2019; Niu et al. 2018; Park et al. 2017; Roy et al. 2021; Ruan et al. 2020; Shi et al. 2016, 2020, 2021; Sinoy et al. 2017; Song et al. 2019; Tajes et al. 2013; Tan et al. 2021; Tang et al. 2013; Tian et al. 2020; Wang et al. 2013, 2018b, c, 2019a, b, c, 2020b; Wang and Xu 2021; Wen et al. 2020; Wu et al. 2020a, b; Xing et al. 2021; Xu et al. 2020b; Yan et al. 2020; Yang et al. 2014, 2021a, b; Yi et al. 2020; Yin et al. 2021; Zappala et al. 2021; Zhang et al. 2016b, c, 2019a, c, 2020b; Zhao et al. 2013; Zhi et al. 2020; Zhou et al. 2019; Zuo et al. 2020). Eight studies found increased cell death rates after ischemia/hypoxia in differentiated SH-SY5Y cells (Cheng et al. 2019, 2014; Fu et al. 2020; Juntunen et al. 2020; Pan et al. 2020; Sriwastva et al. 2020; Xu et al. 2020a; Zhang et al. 2019b). Four studies found increased cell death rates after ischemia/hypoxia in differentiated and undifferentiated SK-N-SH cell model of cerebral ischemia (Ingrassia et al. 2012; Jin et al. 2020; Yanagita et al. 2005; Yin et al. 2021).
Apoptosis is a possible pathway to cell death. Various pro- and anti-apoptotic factors have been investigated. Apoptosis can be triggered through an intrinsic pathway which is regulated by B-cell lymphoma 2 (BCL-2) protein family and is activated by internal signals. The expression of anti-apoptotic BCL-2 has been investigated in 25 studies using undifferentiated SH-SY5Y cells (Chang et al. 2017; Dong et al. 2019; Feng et al. 2021; Gao et al. 2020; Guo 2019; He et al. 2014; Hu et al. 2020; Huang et al. 2018; Li et al. 2019b, 2020a; Liu et al. 2018, 2020; Wang et al. 2013, 2019c, 2020b, 2021a, b; Wu et al. 2020a; Xu et al. 2020b; Yan et al. 2020; Zhang et al. 2016b; Zhao and Wang 2020; Zhi et al. 2020; Zhou et al. 2019) and in one study using differentiated SH-SY5Y cells (Sriwastva et al. 2020). It was found that BCL-2 decreased during and after ischemia/hypoxia. One study investigated the effect of hypoxia on phosphorylated BCL-2 in undifferentiated SH-SY5Y cells and showed increased levels of phosphorylated BCL-2, which could induce autophagic cell death (Wang et al. 2019c). A second member of the BCL-2 family, B-cell lymphoma-extra-large (BCL-XL), an anti-apoptotic protein like BCL-2, prevents apoptosis by preventing the release of small mitochondrial molecules like cytochrome-c (cyt-c). BCL-XL expression was inhibited by ischemia/hypoxia in undifferentiated SH-SY5Y cell models (n = 4) (Castri et al. 2014; He et al. 2014; Li et al. 2020a; Wang et al. 2021b). Besides BCL-2 and BCL-XL, a third anti-apoptotic factor of the BCL-2 family has been investigated, namely Bcl-2-like protein 2 (BCL-W). BCL-W decreased during hypoxia in differentiated SH-SY5Y cells (n = 1) (Xu et al. 2020a).
In addition to anti-apoptotic factors, the BCL-2 family has members that act as pro-apoptotic factors, such as BCL2 associated X (BAX). Expression of this pro-apoptotic protein increased in undifferentiated SH-SY5Y cell models of cerebral ischemia (n = 23) (Castri et al. 2014; Dong et al. 2019; Feng et al. 2021; Gao et al. 2020; Guo 2019; He et al. 2014; Hu et al. 2020; Huang et al. 2018; Li et al. 2019b, 2020a; Liu et al. 2018, 2020; Wang et al. 2013, 2018a, 2019c, 2021b; Wu et al. 2020a; Xu et al. 2020b; Yan et al. 2020; Zhang et al. 2016b; Zhao and Wang 2020; Zhou et al. 2019). Expression of BAX is only investigated in one study using differentiated SH-SY5Y cells. These results showed increased expression of BAX during and after hypoxia (Sriwastva et al. 2020). BAX induces cell death via mitochondrial membrane permeabilization. This results in the release of small molecules such as cyt-c and apoptosis inducing factor (AIF), among others.
Release of cyt-c is investigated in four studies in undifferentiated SH-SY5Y cells (Hu et al. 2020; Li et al. 2020a; Lin et al. 2020; Wang et al. 2019b). The results showed increased cyt-c release, which led to increased apoptotic rate. Bcl2-interacting killer (BIK) enhances apoptosis, it was found to be upregulated in undifferentiated SH-SY5Y cells (Chen et al. 2019). Another pro-apoptotic protein of the BCL-2 family has been investigated in undifferentiated SH-SY5Y cells, namely Bcl-2-like protein 11 (BIM). The expression of BIM was found to be increased during ischemia/hypoxia (n = 2) (He et al. 2014; Song et al. 2019). In addition to BIM, apoptosis facilitator Bcl-2-like protein 14 (BCL2L14) has been investigated in undifferentiated SH-SY5Y cells and the results showed increased expression, which induced apoptosis during ischemia/hypoxia (Yao et al. 2019).
The BCL-2 family induces or prevents the release of apoptogenic factors, which can lead to the activation of the caspase pathway. The caspase family is extensively investigated in relation to apoptosis in ischemia/hypoxia. In studies with undifferentiated SH-SY5Y cells (n = 39), two members of the caspase family (caspase-3 and caspase-9) were increased during and after ischemia/hypoxia (Castri et al. 2014; Chang et al. 2017; Chen et al. 2015, 2019; Dong et al. 2019; Du et al. 2012; Feng et al. 2021; Gao et al. 2020; Hong et al. 2017; Huang et al. 2018; Jia et al. 2021; Li and Ma 2020; Lin et al. 2020; Liu et al. 2018; Marutani et al. 2012; Park et al. 2017; Ruan et al. 2020; Shan et al. 2022; Shi et al. 2020; Song et al. 2019; Tan et al. 2021; Wang et al. 2018a, c, 2019b, 2021a, b; Wen et al. 2020; Wu et al. 2020a; Xing et al. 2021; Xu et al. 2020b; Yan et al. 2020; Yi et al. 2020; Yin et al. 2021; Zappala et al. 2021; Zhang et al. 2016a, b; Zhao and Wang 2020; Zhi et al. 2020; Zuo et al. 2020). Increased expression of caspase-3, -6, -7 after ischemia/hypoxia was found in differentiated SH-SY5Y (n = 5) (Cheng et al. 2014; Sriwastva et al. 2020; Xu et al. 2020a; Zhang et al. 2019b; Zhou et al. 2014). Pro-caspase-9 was found to be negatively correlated with X-linked inhibitor of apoptosis in undifferentiated SH-SY5Y cells (n = 1) (Zhang et al. 2016a). In three studies using undifferentiated SK-N-SH cells, increased expression of caspase-1, -3 and -7 was found after ischemia/hypoxia (Lehane et al. 2013; Yin et al. 2021; Zhou et al. 2016). Similarly, increased expression of caspase-3 was found in one study with differentiated SK-N-SH (Wang et al. 2020a).
AIF translocate to the nucleus upon release by mitochondria, where it triggers chromatin condensation and DNA fragmentation to induce parthanatos. AIF translocation is investigated in undifferentiated SH-SY5Y cells (n = 2). The results showed nuclear translocation of AIF induced by ischemia/hypoxia (Li et al. 2020a; Wang et al. 2018a). One study investigated the expression of AIF in differentiated SH-SY5Y cells and the results showed increased expression induced by hypoxia (Sriwastva et al. 2020). In addition to AIF release from mitochondria, poly(ADP-ribose) polymerase-1 (PARP-1) overactivation mediates AIF release. Normal function of PARP-1 is DNA repair. Cleaved PARP induces parthanatos, which has been investigated in undifferentiated SH-SY5Y cells (n = 5), differentiated SH-SY5Y cells (n = 3), and undifferentiated SK-N-SH (n = 1). Cleaved PARP was increased, which was in turn associated with ischemia/hypoxia induced parthanatos (Han et al. 2017; Lehane et al. 2013; Li et al. 2020a; Ruan et al. 2020; Shi et al. 2020; Sriwastva et al. 2020; Wang et al. 2018a; Xing et al. 2021; Xu et al. 2020a). Endothelial protein C receptor (EPCR) showed anti-cell death features by signaling via protease-activated receptor 1 (PAR1) and PAR3. Levels of these receptors were decreased in differentiated SH-SY5Y cells during and after ischemia/hypoxia (n = 1) (Sriwastva et al. 2020).
Another key player in apoptosis is the tumor suppressor gene P53. This gene is activated by hypoxia and can induce apoptosis. P53 is investigated in one study using undifferentiated SH-SY5Y cells and showed that P53 activity was induced by ischemia/hypoxia (Zhao et al. 2013).
PI3K/AKT/mTOR pathway is an intracellular signaling pathway that plays a role in regulating the cell cycle and apoptosis. This has been investigated in sixteen studies using undifferentiated SH-SY5Y cell models of cerebral ischemia. Overall, a decrease in phosphorylated protein kinase B (AKT), phosphorylated phosphoinositide 3-kinases (PI3K), mTOR and forkhead box proteins (FOXO) were found (Feng et al. 2020; Gao et al. 2020; Guo et al. 2018; Li et al. 2019b; Liu et al. 2019b, 2021; Marutani et al. 2012; Song et al. 2019; Tian et al. 2020; Wang et al. 2019b; Wen et al. 2020; Wu et al. 2020a; Yi et al. 2020; Zhi et al. 2020; Zhou et al. 2019). Decreased levels of AKT were also found in two studies using differentiated SH-SY5Y cell models (Cheng et al. 2019; Han et al. 2017). Epidermal growth factor receptor showed anti-apoptotic effects by activation of the PI3K/AKT pathway in one study with undifferentiated SH-SY5Y and one study with differentiated SH-SY5Y cell models of cerebral ischemia (Lin et al. 2011; Wu et al. 2020b). Glycogen synthase kinase 3β (GSK-3β) was found to be dependent on several signaling pathways, like PI3K/AKT. One study in differentiated SH-SY5Y cells found that increased levels of GSK-3β led to increased apoptotic rate (Lin et al. 2011). Upregulation of phosphatase and tensin homolog (PTEN) was found in four undifferentiated SH-SY5Y and one undifferentiated SK-N-SH cell models of cerebral ischemia (Gao et al. 2020; Guo 2019; Guo et al. 2018; Jin et al. 2020; Yi et al. 2020).
Some other factors that are associated with cell death and have been investigated in human neuronal in vitro models of cerebral ischemia are Sphingosine kinase 2 (decreased with ischemia in undifferentiated SH-SY5Y cells) (n = 1) (Di et al. 2017), death-associated protein kinase 1 (DAPK1; elevated with ischemia in undifferentiated SH-SY5Y cells) (n = 1; (Feng et al. 2021)), heat shock protein20 (HSP20; decreased and initiated Golgi apparatus fragmentation) (n = 1) (Lu et al. 2019), and matrix metallopeptidase 9 (MMP-9; increased macrophage migration inhibitory factor (MIF), which is recruited to cleave DNA) (n = 1) (Li et al. 2020a).
Increased nuclear fragmentation has been found in undifferentiated SH-SY5Y cell model (n = 1) (Liu et al. 2019a). FMS-like tyrosine kinase-3 (FLT3) has been implicated in cell survival. One study into this kinase showed that FLT3 expression increased in undifferentiated SH-SY5Y cell model of cerebral ischemia (Dong et al. 2019).
Toll-like receptor 8 (TLR8) was correlated with increased cell death rate in an undifferentiated SH-SY5Y cell model (Tang et al. 2013). Levels of brain derived neurotrophic factor (BDNF) showed no changes during ischemia/hypoxia in undifferentiated SH-SY5Y cells (n = 3) (Chang et al. 2017; Fang et al. 2020; Wang et al. 2013). The MAPK/JNK/ERK signaling pathway has been implicated in regulation of cell death in differentiated (n = 4) (Cheng et al. 2014; Chi et al. 2019; Xu et al. 2020a; Zeng et al. 2019) and undifferentiated SH-SY5Y (n = 13) cell models of cerebral ischemia (He et al. 2014; Liu et al. 2019a; Marutani et al. 2012; Ruan et al. 2021; Shi et al. 2020; Tian et al. 2020; Wang et al. 2013, 2017, 2019c; Yang et al. 2014). Interferon regulatory factor-1 (IRF-1) has been investigated in relation to cell death and results showed a correlation between increased IRF-1 and cell death in undifferentiated SH-SY5Y cell model of cerebral ischemia (n = 1) (Liu et al. 2020).
The pro-apoptotic factor ‘programmed cell death 4’ (PDCD4) was found to be increased in an undifferentiated SH-SY5Y cell model of cerebral ischemia (Shan et al. 2022). Increased expression of inhibitor of growth protein 5 (ING5) was correlated with an increased apoptotic rate in an undifferentiated SH-SY5Y cell model of cerebral ischemia (Zhang et al. 2019a). Hypoxia-inducible factor-1 showed to be upregulated, followed by downregulation during ischemia/hypoxia and played a role in ischemia/hypoxia induced cell death in undifferentiated SH-SY5Y cells (n = 4) and SK-N-SH cells (n = 1) (Lin-Holderer et al. 2016; Niu et al. 2018; Olechnowicz et al. 2012; Ruan et al. 2021; Zhang et al. 2016c). Modulation of apoptosis-1 is an apoptotic pathway that was found to be increased in undifferentiated SH-SY5Y cells (n = 1) (Chan et al. 2019).
Cell membrane damage has been investigated in differentiated and undifferentiated SH-SY5Y cell models (n = 28) (Chan et al. 2015; Chen et al. 2019; Cheng et al. 2014, 2019; Chi et al. 2019; Gao et al. 2020; Hsieh et al. 2021; Li and Ma 2020; Lin et al. 2011; Luan et al. 2013; Marutani et al. 2012; Nampoothiri and Rajanikant 2019; Park et al. 2017; Ruan et al. 2020; Sriwastva et al. 2020; Wang et al. 2018a, 2019b, c; Wang and Xu 2021; Wu et al. 2020a, b; Xing et al. 2021; Xu et al. 2020a; Zeng et al. 2019; Zhang et al. 2016c, 2019b; Zhao et al. 2013; Zhi et al. 2020) and differentiated and undifferentiated SK-N-SH cell models (n = 3) (Ingrassia et al. 2012; Lehane et al. 2013; Rosenthal et al. 2017).
NT2-N Cell Model
Two studies investigated ischemia/hypoxia in differentiated NTERA-2 clone D1 (NT2-N) cell model. These studies investigated complement system activation upon ischemia/hypoxia induced stress. The complement system is part of the immune system that promotes inflammation. The first study into expression of several complement regulators, such as CD35, CD45, CD55, CD59, C3a and C5a, showed decreased expression of CD55 and an increased vulnerability to hypoxia (Pedersen et al. 2007b).
The second study aimed to investigate whether complement regulatory protein CD59 was expressed in the NT2-N cell model and what its function was. The results showed that CD59 was expressed and that this protein played a role in protecting the cells from complement attack and eventually cell death (Pedersen et al. 2007a).
hiPSC-Derived Neuronal Cell Model
Two studies investigated ischemia/hypoxia in healthy hiPSC-derived neuronal cell models. One study investigated hiPSC-derived neuronal networks consisting of excitatory and inhibitory neurons. Electrophysiological measurements showed decreased neuronal activity, as well as decreased synchronous activity, throughout the network. Furthermore, the results showed increased levels of apoptosis on time scales of 24–48 h of hypoxia, followed by an increased level of dead cells (Pires Monteiro et al. 2021).
The other study compared the effect of ischemia/hypoxia on hiPSC-derived neurons with that on differentiated SH-SY5Y cells. The results showed less cell survival of SH-SY5Y cells than of hiPSC-derived neurons (Juntunen et al. 2020).
Treatment Targets
Several steps of the ischemic cascade have been targeted by pharmacological treatment strategies to prevent irreversible neuronal damage. Thirty-six studies in undifferentiated SH-SY5Y cell models (Chen et al. 2021b; Dong et al. 2021; Gao et al. 2017; Hao et al. 2015; Hong et al. 2017; Hsieh et al. 2021; Huang et al. 2021; Janssen et al. 2021; Jiang et al. 2012; Jimenez-Almarza et al. 2019; Lai et al. 2020; Landgraf et al. 2020; Li et al. 2019a, b, 2020a; Lin-Holderer et al. 2016; Liu et al. 2016, 2019a, b; Liu 2016; Luan et al. 2013; Marutani et al. 2012; Park et al. 2017; Ruan et al. 2021; Shi et al. 2019; Song et al. 2019; Wang et al. 2013, 2019b, c, 2021a; Wu et al. 2020a, b; Xu et al. 2020b; Yang et al. 2014; Zappala et al. 2021; Zhi et al. 2020), seven in differentiated SH-SY5Y cell models (Cheng et al. 2014, 2019; Fu et al. 2020; Lin et al. 2011; Sriwastva et al. 2020; Zeng et al. 2019; Zhang et al. 2019b), two in undifferentiated SK-N-SH cell models (Lehane et al. 2013; Soh et al. 2007), and one in hiPSC-derived neurons (Pires Monteiro et al. 2021) included treatment with a pharmacological compound. Treatment targets were neuronal activity, oxidative/nitrosative stress, inflammation, autophagy or cell death.
One study with hiPSC-derived neurons investigated the effect of neuronal network activation by the mildly excitatory hormone/neurotransmitter ghrelin (Pires Monteiro et al. 2021). The results showed partial preservation of neuronal network functioning after hypoxia (Pires Monteiro et al. 2021).
Oxidative/nitrosative stress was investigated as a treatment target in twenty studies using undifferentiated SH-SY5Y cells and one study using differentiated SH-SY5Y cells. These studies showed that various chemical compounds could reduce oxidative stress by reducing the formation of ROS or increasing anti-oxidant defense mechanisms, which led to increased cell survival (Agudo-Lopez et al. 2010; Chen et al. 2021b; Dong et al. 2021; Gao et al. 2017; Hong et al. 2017; Hsieh et al. 2021; Jimenez-Almarza et al. 2019; Landgraf et al. 2020; Li et al. 2020a; Liu et al. 2019a; Liu 2016; Marutani et al. 2012; Park et al. 2017; Ruan et al. 2021; Shi et al. 2019; Sriwastva et al. 2020; Wang et al. 2019b; Wu et al. 2020a; Xu et al. 2020b; Yang et al. 2021b; Zhi et al. 2020).
Inflammation was investigated as a treatment target in twelve studies using undifferentiated SH-SY5Y cells and in one study using differentiated SH-SY5Y cells. These studies showed that various chemical compounds could decrease several pro-inflammatory factors (NF-κB, TNF, IL-6, IL-B1) to alleviate inflammation (Chen et al. 2015; Dong et al. 2021; Hao et al. 2015; Huang et al. 2021; Janssen et al. 2021; Jiang et al. 2012; Landgraf et al. 2020; Li et al. 2019a, b; Ruan et al. 2021; Yang et al. 2014; Zhi et al. 2020).
Autophagy was investigated as a treatment target in five studies using undifferentiated SH-SY5Y cells and two studies using differentiated SH-SY5Y cells (Cheng et al. 2019; Lai et al. 2020; Liu 2016; Liu et al. 2016; Wang et al. 2019c; Zhang et al. 2019b; Zhi et al. 2020). These studies showed that various pharmacological compounds could maintain healthy autophagic flux.
Apoptosis was investigated as a treatment target in 26 studies using undifferentiated SH-SY5Y cells, four studies using differentiated SH-SY5Y cells, and two studies using undifferentiated SK-N-SH cells. These studies showed that pharmacological compounds could decrease apoptosis, by reducing expression of pro-apoptotic factors and increasing expression of anti-apoptotic factors, which led to increased cell survival (Agudo-Lopez et al. 2010; Chen et al. 2021b; Cheng et al. 2014; Dong et al. 2021; Gao et al. 2017; Hao et al. 2015; Huang et al. 2021; Jiang et al. 2012; Li et al. 2019b, 2020a; Lin et al. 2011, 2020; Liu et al. 2019a, b; Luan et al. 2013; Marutani et al. 2012; Park et al. 2017; Song et al. 2019; Sriwastva et al. 2020; Wang et al. 2013, 2019b, c, 2021a; Wu et al. 2020a, b; Xu et al. 2020b; Yang et al. 2014; Zappala et al. 2021; Zhang et al. 2019b; Zhi et al. 2020).
Discussion
With this scoping review, we provide an overview of human neuronal in vitro models that have been used to study human neuronal responses to ischemia or hypoxia, including the established steps in the pathophysiological cascade and potential treatment targets. The scoping review shows that models based on SH-SY5Y cells are the most widely used in this field. Almost all studies used immunocytochemical techniques to investigate cell survival and protein expression. Neuronal functioning was investigated in only one study using hiPSC-derived neuronal networks. Cell death was the most investigated pathomechanism, followed by oxidative stress and inflammation. Studies on apoptosis revealed the importance of diverse pro- and anti-apoptotic factors such as the BCL-2 protein family that lead to apoptosis and eventually cell death. Studies on oxidative stress showed higher levels of ROS and lower levels of ROS scavenging molecules after ischemia or hypoxia. The most important results on inflammatory responses were increased levels of pro-inflammatory and decreased levels of anti-inflammatory cytokines.
SH-SY5Y cells are a subclone from SK-N-SH, originating from a single 4-year-old female neuroblastoma patient (Biedler et al. 1973). The wide spread use of SH-SY5Y cells indicates that pathomechanisms of human neuronal responses to ischemia or hypoxia have mainly been investigated in a cancerous cell line (Liu et al. 2018). It is known that the genetic background of a cell line affects functionality, as well as responses to interventions (Mossink et al. 2021b). Thus, a cell line with cancerous characteristics will probably respond to ischemia or hypoxia differently than healthy (neuronal) cells.
Moreover, although SH-SY5Y cells can be differentiated into neuron-like cells upon stimulation with RA (Khwanraj et al. 2015; Korecka et al. 2013; Shipley et al. 2016), the majority of studies has been conducted in undifferentiated SH-SY5Y cells. Undifferentiated and differentiated SH-SY5Y cells differ in morphology and function. Undifferentiated SH-SY5Y cells have large cell bodies with short truncated processes (Kovalevich and Langford 2013). These undifferentiated cells express immature neuronal markers, such as SOX2, and lack the expression of mature neuronal markers, such as synapsin1/2 positive puncta. Differentiated SH-SY5Y cells form and extent neuritic processes (Kovalevich and Langford 2013) and show increased expression of synaptic markers, such as SNAP25 and SYN1 (Forster et al. 2016). Differentiated SH-SY5Y cells acquired the neuronal ability to produce trains of spikes upon prolonged stimulation in current-clamp experiments (Tosetti et al. 1998) and showed spontaneous firing, bursting, and network behaviour on micro-electrode arrays (MEAs) (Yoon et al. 2020), that were not observed in undifferentiated SH-SY5Y cells. These differences may lead to divergent responses to ischemia/hypoxia between undifferentiated and differentiated SH-SY5Y cells. Thus, it is questionable whether results from studies in undifferentiated SH-SY5Y cells can be extrapolated to neurons or neuronal networks.
OGD directly models ischemic stress and allows for the exploration of divergent components of the pathophysiological cascade caused by ischemic stress. Furthermore, modelling of OGD is straightforward and can be replicated in various laboratories (Cimarosti and Henley 2008). Other proposed cerebral ischemia models are based on chemical or enzymatic modulation of specific pathophysiological pathways. These can be used to study specific components of the ischemic cascade, such as excitotoxicity or glutathione depletion. These techniques are commonly used to investigate the effect of increased glutamate release or induced oxidative stress caused by ischemic stroke, respectively (Nicholls and Ward 2000). In turn, these may lead to other downstream effects, for example calcium dysregulation (by excitotoxicity) or glutathione depletion (by oxidative stress) (Mattson et al. 1995; Mytilineou et al. 1998). However, it is important to note that these techniques probably model only parts of the ischemic stress cascade (Cimarosti and Henley 2008). Moreover, it is unclear whether chemical or enzymatic induction of downstream effects has the desired effect on undifferentiated SH-SY5Y cells, which are often used in ischemic stroke research. For example, none of the studies included in this scoping review touched upon excitotoxicity. This highlights the need for a careful assessment of the suitability of the various cell models and modes of ischemic stress induction in light of the research question at hand.
The majority of human neuronal in vitro studies on effects of ischemia/hypoxia focused on cell survival and protein expression. Expression of proteins that might have deleterious effects (such as pro-apoptotic factors, pro-inflammatory cytokines, oxidative stress factors) apparently increase and expression of proteins that might have protective effects (such as anti-apoptotic factors and anti-inflammatory factors) decrease. Various studies showed this in relationship to cell death, apoptosis, oxidative stress, or inflammation. This focus on microscopic measures of relatively downstream processes neglects early steps in the pathophysiological cascade of ischemia, such as energy failure caused by OGD (Galkin 2019), loss of cell ion homeostasis (Caplan 2009; Taoufik and Probert 2008), and depression of excitatory synaptic transmission. It also neglects effects on neuronal functionality and activity (Bolay et al. 2002; Hofmeijer and van Putten 2012; Pires Monteiro et al. 2021).
HiPSC-derived neuronal networks on MEAs are currently the only functional human neuronal cell model used to investigate neuronal network activity in cerebral ischemia (Pires Monteiro et al. 2021). HiPSCs can be derived from healthy individuals or patients and differentiated in excitatory neurons and inhibitory neurons. This allows establishment of healthy neuronal networks with physiological excitatory – inhibitory (E/I) ratio’s (Frega et al. 2017; Mossink et al. 2021a). Genetic characteristics of the donor are preserved in the model, making it possible to study patient-specific network behaviour, including responses to ischemia/hypoxia (Mossink et al. 2021b). By the use of MEAs, functionality of many neurons and synapses in the network can be studied simultaneously. Despite the lack of normal brain architecture, neuronal network functioning can be readily derived, in addition to microscopic measures. The dynamics of neuronal network failure, from reversible to irreversible damage or recovery, can be monitored continuously (Pires Monteiro et al. 2021). This allows identification of early determinants of irreversibility, including possible effects of interventions.
Studies on interventions to ameliorate pathophysiological processes during or after ischemia/hypoxia focused on a wide range of treatment targets. Evidence from studies in SH-SY5Y cells mostly suggests effects of treatments targeting apoptosis or other modes of cell death, because this was the most targeted pathomechanism (Agudo-Lopez et al. 2010; Chen et al. 2021b; Cheng et al. 2014; Dong et al. 2021; Gao et al. 2017; Hao et al. 2015; Huang et al. 2021; Jiang et al. 2012; Li et al. 2019b, 2020a; Lin et al. 2011, 2020; Liu et al. 2019a, b; Luan et al. 2013; Marutani et al. 2012; Park et al. 2017; Song et al. 2019; Sriwastva et al. 2020; Wang et al. 2013, 2019b, c, 2021a; Wu et al. 2020a, b; Xu et al. 2020b; Yang et al. 2014; Zappala et al. 2021; Zhang et al. 2019b; Zhi et al. 2020). One study in hiPSC-derived neuronal networks on MEAs showed beneficial effects of early neuronal network stimulation on functional network recovery and cell survival (Pires Monteiro et al. 2021). All intervention studies were small sampled proof of principle experiment and none of the intervention effects was conformed in follow-up studies.
In vitro models of OGD provide a versatile platform for replicating diverse aspects of ischemic stroke pathology in a reproducible manner. Compared to in vivo models, these models are often quicker, cheaper and easier to establish. Also, the use of human-derived models may contribute to a reduction of animal model use. However, for clinical translation, combinations of in vitro and in vivo models will continue to be necessary (Trotman-Lucas and Gibson 2021). With the increasing amount of modelling possibilities, it will remain important to consider the optimal model based on the research goal at hand.
The strengths of this scoping review are the prospective literature research and systematic data extraction. Limitations include that the review was not pre-registered, data extraction was conducted by only one reviewer (EV), and lack of quality assessment of the included articles. Data on effort, costs, and reproducibility of the models are not systematically presented in the included research papers.
In conclusion, the majority of human neuronal in vitro studies on experimental cerebral ischemia/hypoxia exploited the cancerous SH-SY5Y cell line, mostly in a relatively immature stage and not differentiated into neuronal-like cells. Most studies focused on microscopic measures of single downstream pathophysiological processes, such as cell death. Intervention studies targeted a myriad of treatment targets and none of the intervention effects presented in this review have been confirmed in follow-up experiments. We suggest the use of networks of differentiated neurons on MEAs. These provide new opportunities to analyze neuronal network functioning, in addition to microscopic measures. This allows coupling of neuronal structure and function (which is not possible in unfunctional neurons) in order to identify drivers of neuronal network (dys)functioning. The use of hiPSC-derived neuronal networks provides additional opportunities to study networks with physiological E/I ratios and identify person-specific characteristics. For intervention studies, we favor standardization to collect strong evidence of (in)efficacy to improve translation from bench to bedside.
Data Availability
Enquiries about data availability should be directed to the authors.
References
Agudo-Lopez A, Miguel BG, Fernandez I, Martinez AM (2010) Involvement of mitochondria on neuroprotective effect of sphingosine-1-phosphate in cell death in an in vitro model of brain ischemia. Neurosci Lett 470(2):130–133. https://doi.org/10.1016/j.neulet.2009.12.070
Beske PH, Jackson DA (2012) NADPH oxidase mediates the oxygen-glucose deprivation/reperfusion-induced increase in the tyrosine phosphorylation of the N-methyl-d-aspartate receptor NR2A subunit in retinoic acid differentiated SH-SY5Y Cells. J Mol Signal 7(1):15. https://doi.org/10.1186/1750-2187-7-15
Biedler JL, Helson L, Spengler BA (1973) Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res 33(11):2643–2652
Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS (1978) Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res 38(11 Pt 1):3751–3757
Bolay H, Gursoy-Ozdemir Y, Sara Y, Onur R, Can A, Dalkara T (2002) Persistent defect in transmitter release and synapsin phosphorylation in cerebral cortex after transient moderate ischemic injury. Stroke 33(5):1369–1375. https://doi.org/10.1161/01.str.0000013708.54623.de
Caplan L (2009) Caplan’s stroke: a clinical approach, 4th edn. Saunders, Philadelphia
Castri P, Lee YJ, Ponzio T, Maric D, Spatz M, Bembry J, Hallenbeck J (2014) Poly(ADP-ribose) polymerase-1 and its cleavage products differentially modulate cellular protection through NF-kappaB-dependent signaling. Biochim Biophys Acta 1843(3):640–651. https://doi.org/10.1016/j.bbamcr.2013.12.005
Chai Z, Gong J, Zheng P, Zheng J (2020) Inhibition of miR-19a-3p decreases cerebral ischemia/reperfusion injury by targeting IGFBP3 in vivo and in vitro. Biol Res 53(1):17. https://doi.org/10.1186/s40659-020-00280-9
Chan SJ, Chai C, Lim TW, Yamamoto M, Lo EH, Lai MK, Wong PT (2015) Cystathionine beta-synthase inhibition is a potential therapeutic approach to treatment of ischemic injury. ASN Neuro. https://doi.org/10.1177/1759091415578711
Chan SJ, Zhao H, Hayakawa K, Chai C, Tan CT, Huang J, Tao R, Hamanaka G, Arumugam TV, Lo EH, Yu VCK, Wong PH (2019) Modulator of apoptosis-1 is a potential therapeutic target in acute ischemic injury. J Cereb Blood Flow Metab 39(12):2406–2418. https://doi.org/10.1177/0271678X18794839
Chang CF, Lai JH, Wu JC, Greig NH, Becker RE, Luo Y, Chen YH, Kang SJ, Chiang YH, Chen KY (2017) (−)-Phenserine inhibits neuronal apoptosis following ischemia/reperfusion injury. Brain Res 1677:118–128. https://doi.org/10.1016/j.brainres.2017.09.015
Chen SHC (2004) Neuropeptide Y-Y1 receptor agonist worsens while antagonist improves survival of cultured Y1-expressing neuronal cells following oxygen and glucose deprivation. J Biomed Sci 11:781–788. https://doi.org/10.1159/000081825
Chen B, Wu Z, Xu J, Xu Y (2015) Calreticulin binds to Fas ligand and inhibits neuronal cell apoptosis induced by ischemia-reperfusion injury. Biomed Res Int 2015:895284. https://doi.org/10.1155/2015/895284
Chen X, Li C, Li J, Sheng L, Liu X (2019) Upregulation of miR-1306-5p decreases cerebral ischemia/reperfusion injury in vitro by targeting BIK. Biosci Biotechnol Biochem 83(12):2230–2237. https://doi.org/10.1080/09168451.2019.1654846
Chen W, Wang L, Liu Z (2021a) MicroRNA-155 influences cell damage in ischemic stroke via TLR4/MYD88 signaling pathway. Bioengineered 12(1):2449–2458. https://doi.org/10.1080/21655979.2021.1935066
Chen Y, Fan Z, Wu Q (2021b) Dexmedetomidine improves oxygen-glucose deprivation/reoxygenation (OGD/R) -induced neurological injury through regulating SNHG11/miR-324-3p/VEGFA axis. Bioengineered 12(1):4794–4804. https://doi.org/10.1080/21655979.2021.1957071
Cheng YL, Choi Y, Seow WL, Manzanero S, Sobey CG, Jo DG, Arumugam TV (2014) Evidence that neuronal Notch-1 promotes JNK/c-Jun activation and cell death following ischemic stress. Brain Res 1586:193–202. https://doi.org/10.1016/j.brainres.2014.08.054
Cheng A, Lu Y, Huang Q, Zuo Z (2019) Attenuating oxygen-glucose deprivation-caused autophagosome accumulation may be involved in sevoflurane postconditioning-induced protection in human neuron-like cells. Eur J Pharmacol 849:84–95. https://doi.org/10.1016/j.ejphar.2019.01.051
Chi L, Jiao D, Nan G, Yuan H, Shen J, Gao Y (2019) miR-9-5p attenuates ischemic stroke through targeting ERMP1-mediated endoplasmic reticulum stress. Acta Histochem 121(8):151438. https://doi.org/10.1016/j.acthis.2019.08.005
Cimarosti H, Henley JM (2008) Investigating the mechanisms underlying neuronal death in ischemia using in vitro oxygen-glucose deprivation: potential involvement of protein SUMOylation. Neuroscientist 14(6):626–636. https://doi.org/10.1177/1073858408322677
Di G, Wang Z, Wang W, Cheng F, Liu H (2017) AntagomiR-613 protects neuronal cells from oxygen glucose deprivation/re-oxygenation via increasing SphK2 expression. Biochem Biophys Res Commun 493(1):188–194. https://doi.org/10.1016/j.bbrc.2017.09.049
Di G, Yang X, Cheng F, Liu H, Xu M (2021) CEBPA-AS1 knockdown alleviates oxygen-glucose deprivation/reperfusion-induced neuron cell damage by the MicroRNA 24–3p/BOK axis. Mol Cell Biol 41(8):e0006521. https://doi.org/10.1128/MCB.00065-21
Dong RF, Tai LW, Zhang B, Shi FK, Liu HM, Duan PC, Cheng Y (2019) Neuroprotective effect of FMS-like tyrosine kinase-3 silence on cerebral ischemia/reperfusion injury in a SH-SY5Y cell line. Gene 697:152–158. https://doi.org/10.1016/j.gene.2019.01.043
Dong X, Wang L, Song G, Cai X, Wang W, Chen J, Wang G (2021) Physcion protects rats against cerebral ischemia-reperfusion injury via inhibition of TLR4/NF-kB signaling pathway. Drug Des Dev Ther 15:277–287. https://doi.org/10.2147/DDDT.S267856
Du CP, Tan R, Hou XY (2012) Fyn kinases play a critical role in neuronal apoptosis induced by oxygen and glucose deprivation or amyloid-beta peptide treatment. CNS Neurosci Ther 18(9):754–761. https://doi.org/10.1111/j.1755-5949.2012.00357.x
Fang YC, Chan L, Liou JP, Tu YK, Lai MJ, Chen CI, Vidyanti AN, Lee HY, Hu CJ (2020) HDAC inhibitor protects chronic cerebral hypoperfusion and oxygen-glucose deprivation injuries via H3K14 and H4K5 acetylation-mediated BDNF expression. J Cell Mol Med 24(12):6966–6977. https://doi.org/10.1111/jcmm.15358
Feng H, Hu L, Zhu H, Tao L, Wu L, Zhao Q, Gao Y, Gong Q, Mao F, Li X, Zhou H, Li J, Zhang H (2020) Repurposing antimycotic ciclopirox olamine as a promising anti-ischemic stroke agent. Acta Pharm Sin B 10(3):434–446. https://doi.org/10.1016/j.apsb.2019.08.002
Feng M, Zhu X, Zhuo C (2021) H19/miR-130a-3p/DAPK1 axis regulates the pathophysiology of neonatal hypoxic-ischemia encephalopathy. Neurosci Res 163:52–62. https://doi.org/10.1016/j.neures.2020.03.005
Formisano L, Guida N, Valsecchi V, Pignataro G, Vinciguerra A, Pannaccione A, Secondo A, Boscia F, Molinaro P, Sisalli MJ, Sirabella R, Casamassa A, Canzoniero LM, Di Renzo G, Annunziato L (2013) NCX1 is a new rest target gene: role in cerebral ischemia. Neurobiol Dis 50:76–85. https://doi.org/10.1016/j.nbd.2012.10.010
Forster JI, Koglsberger S, Trefois C, Boyd O, Baumuratov AS, Buck L, Balling R, Antony PM (2016) Characterization of differentiated SH-SY5Y as neuronal screening model reveals increased oxidative vulnerability. J Biomol Screen 21(5):496–509. https://doi.org/10.1177/1087057115625190
Frega M, van Gestel SH, Linda K, van der Raadt J, Keller J, Van Rhijn JR, Schubert D, Albers CA, Nadif Kasri N (2017) Rapid neuronal differentiation of induced pluripotent stem cells for measuring network activity on micro-electrode arrays. J vis Exp. https://doi.org/10.3791/54900
Fu C, Zhang X, Zeng Z, Tian Y, Jin X, Wang F, Xu Z, Chen B, Zheng H, Liu X (2020) Neuroprotective effects of qingnao dripping pills against cerebral ischemia via inhibiting NLRP3 inflammasome signaling pathway: in vivo and in vitro. Front Pharmacol 11:65. https://doi.org/10.3389/fphar.2020.00065
Galkin A (2019) Brain ischemia/reperfusion injury and mitochondrial complex I damage. Biochemistry (mosc) 84(11):1411–1423. https://doi.org/10.1134/S0006297919110154
Gao GS, Li Y, Zhai H, Bi JW, Zhang FS, Zhang XY, Fan SH (2017) Humanin analogue, S14G-humanin, has neuroprotective effects against oxygen glucose deprivation/reoxygenation by reactivating Jak2/Stat3 signaling through the PI3K/AKT pathway. Exp Ther Med 14(4):3926–3934. https://doi.org/10.3892/etm.2017.4934
Gao N, Tang H, Gao L, Tu GL, Luo H, Xia Y (2020) LncRNA H19 aggravates cerebral ischemia/reperfusion injury by functioning as a ceRNA for miR-19a-3p to target PTEN. Neuroscience 437:117–129. https://doi.org/10.1016/j.neuroscience.2020.04.020
Ginsberg MD (2008) Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology 55(3):363–389. https://doi.org/10.1016/j.neuropharm.2007.12.007
Guo S, Miyake M, Liu KJ, Shi H (2009) Specific inhibition of hypoxia inducible factor 1 exaggerates cell injury induced by in vitro ischemia through deteriorating cellular redox environment. J Neurochem 108(5):1309–1321. https://doi.org/10.1111/j.1471-4159.2009.05877.x
Guo Y, Wang LP, Li C, Xiong YX, Yan YT, Zhao LQ, Li SD, Sun J, Luo HY, Xian CJ (2018) Effects of ginsenoside Rb1 on expressions of phosphorylation Akt/phosphorylation mTOR/phosphorylation PTEN in artificial abnormal hippocampal microenvironment in rats. Neurochem Res 43(10):1927–1937. https://doi.org/10.1007/s11064-018-2612-x
Guo X-L, Sun Y, Wang C-B (2019) MicroRNA-26a regulates cerebral ischemia injury through targeting PTEN. Eur Rev Med Pharmacol Sci 23:7033–7041
Ham PB 3rd, Raju R (2017) Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog Neurobiol 157:92–116. https://doi.org/10.1016/j.pneurobio.2016.06.006
Han J, Luk B, Lee FJ (2017) Neuroprotective effects of extracellular DJ-1 on reperfusion injury in SH-SY5Y cells. Synapse 71(5):e21963. https://doi.org/10.1002/syn.21963
Hao M, Li X, Feng J, Pan N (2015) Triptolide protects against ischemic stroke in rats. Inflammation 38(4):1617–1623. https://doi.org/10.1007/s10753-015-0137-x
He C, Stroink AR, Wang CX (2014) The role of DAPK-BimEL pathway in neuronal death induced by oxygen-glucose deprivation. Neuroscience 258:254–262. https://doi.org/10.1016/j.neuroscience.2013.11.024
Herrmann AG, Deighton RF, Le Bihan T, McCulloch MC, Searcy JL, Kerr LE, McCulloch J (2013) Adaptive changes in the neuronal proteome: mitochondrial energy production, endoplasmic reticulum stress, and ribosomal dysfunction in the cellular response to metabolic stress. J Cereb Blood Flow Metab 33(5):673–683. https://doi.org/10.1038/jcbfm.2012.204
Hofmeijer J, van Putten MJ (2012) Ischemic cerebral damage: an appraisal of synaptic failure. Stroke 43(2):607–615. https://doi.org/10.1161/STROKEAHA.111.632943
Hong S, Kwon J, Kim DW, Lee HJ, Lee D, Mar W (2017) Mulberrofuran G protects ischemic injury-induced cell death via inhibition of NOX4-mediated ROS generation and ER stress. Phytother Res 31(2):321–329. https://doi.org/10.1002/ptr.5754
Hsieh YS, Shin YK, Seol GH (2021) Protection of the neurovascular unit from calcium-related ischemic injury by linalyl acetate. Chin J Physiol 64(2):88–96. https://doi.org/10.4103/cjp.cjp_94_20
Hu L, Fang R, Guo M (2020) Knockdown of lncRNA SNHG1 alleviates oxygen-glucose deprivation/reperfusion-induced cell death by serving as a ceRNA for miR-424 in SH-SY5Y cells. Neurol Res 42(1):47–54. https://doi.org/10.1080/01616412.2019.1672389
Huang C, Zhou H, Ren X, Teng J (2018) Inhibition of JAK1 by microRNA-708 promotes SH-SY5Y neuronal cell survival after oxygen and glucose deprivation and reoxygenation. Neurosci Lett 664:43–50. https://doi.org/10.1016/j.neulet.2017.11.017
Huang L, Shi Y, Zhao L (2021) Ginkgolide B alleviates learning and memory impairment in rats with vascular dementia by reducing neuroinflammation via regulating NF-kappaB pathway. Front Pharmacol 12:676392. https://doi.org/10.3389/fphar.2021.676392
Ingrassia R, Lanzillotta A, Sarnico I, Benarese M, Blasi F, Borgese L, Bilo F, Depero L, Chiarugi A, Spano PF, Pizzi M (2012) 1B/(-)IRE DMT1 expression during brain ischemia contributes to cell death mediated by NF-kappaB/RelA acetylation at Lys310. PLoS ONE 7(5):e38019. https://doi.org/10.1371/journal.pone.0038019
Janssen L, Ai X, Zheng X, Wei W, Caglayan AB, Kilic E, Wang YC, Hermann DM, Venkataramani V, Bahr M, Doeppner TR (2021) Inhibition of fatty acid synthesis aggravates brain injury, reduces blood-brain barrier integrity and impairs neurological recovery in a murine stroke model. Front Cell Neurosci 15:733973. https://doi.org/10.3389/fncel.2021.733973
Jia Y, Liu J, Hu H, Duan Q, Chen J, Li L (2021) MiR-363-3p attenuates neonatal hypoxic-ischemia encephalopathy by targeting DUSP5. Neurosci Res 171:103–113. https://doi.org/10.1016/j.neures.2021.03.003
Jiang WL, Xu Y, Zhang SP, Zhu HB, Hou J (2012) Tricin 7-glucoside protects against experimental cerebral ischemia by reduction of NF-kappaB and HMGB1 expression. Eur J Pharm Sci 45(1–2):50–57. https://doi.org/10.1016/j.ejps.2011.10.019
Jimenez-Almarza A, Diez-Iriepa D, Chioua M, Chamorro B, Iriepa I, Martinez-Murillo R, Hadjipavlou-Litina D, Oset-Gasque MJ, Marco-Contelles J (2019) Synthesis, neuroprotective and antioxidant capacity of PBN-related indanonitrones. Bioorg Chem 86:445–451. https://doi.org/10.1016/j.bioorg.2019.01.071
Jin X, Wang H, Yin S, Zhang Y (2020) MicroRNA-19a mediates neuroprotection through the PTEN/AKT pathway in SK-N-SH cells after oxygen-glucose deprivation/reoxygenation injury. Gen Physiol Biophys 39(3):259–268. https://doi.org/10.4149/gpb_2020001
Juntunen M, Hagman S, Moisan A, Narkilahti S, Miettinen S (2020) In vitro oxygen-glucose deprivation-induced stroke models with human neuroblastoma cell- and induced pluripotent stem cell-derived neurons. Stem Cells Int 2020:8841026. https://doi.org/10.1155/2020/8841026
Karuppagounder SS, Basso M, Sleiman SF, Ma TC, Speer RE, Smirnova NA, Gazaryan IG, Ratan RR (2013) In vitro ischemia suppresses hypoxic induction of hypoxia-inducible factor-1alpha by inhibition of synthesis and not enhanced degradation. J Neurosci Res 91(8):1066–1075. https://doi.org/10.1002/jnr.23204
Khwanraj K, Phruksaniyom C, Madlah S, Dharmasaroja P (2015) Differential expression of tyrosine hydroxylase protein and apoptosis-related genes in differentiated and undifferentiated SH-SY5Y neuroblastoma cells treated with MPP(.). Neurol Res Int 2015:734703. https://doi.org/10.1155/2015/734703
Korecka JA, van Kesteren RE, Blaas E, Spitzer SO, Kamstra JH, Smit AB, Swaab DF, Verhaagen J, Bossers K (2013) Phenotypic characterization of retinoic acid differentiated SH-SY5Y cells by transcriptional profiling. PLoS ONE 8(5):e63862. https://doi.org/10.1371/journal.pone.0063862
Kovalevich J, Langford D (2013) Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol 1078:9–21. https://doi.org/10.1007/978-1-62703-640-5_2
Lai Z, Gu L, Yu L, Chen H, Yu Z, Zhang C, Xu X, Zhang M, Zhang M, Ma M, Zhao Z, Zhang J (2020) Delta opioid peptide [d-Ala2, d-Leu5] enkephalin confers neuroprotection by activating delta opioid receptor-AMPK-autophagy axis against global ischemia. Cell Biosci 10:79. https://doi.org/10.1186/s13578-020-00441-z
Landgraf AD, Alsegiani AS, Alaqel S, Thanna S, Shah ZA, Sucheck SJ (2020) Neuroprotective and anti-neuroinflammatory properties of Ebselen derivatives and their potential to inhibit neurodegeneration. ACS Chem Neurosci 11(19):3008–3016. https://doi.org/10.1021/acschemneuro.0c00328
Lee OH, Kim J, Kim JM, Lee H, Kim EH, Bae SK, Choi Y, Nam HS, Heo JH (2013) Decreased expression of sirtuin 6 is associated with release of high mobility group box-1 after cerebral ischemia. Biochem Biophys Res Commun 438(2):388–394. https://doi.org/10.1016/j.bbrc.2013.07.085
Lehane C, Guelzow T, Zenker S, Erxleben A, Schwer CI, Heimrich B, Buerkle H, Humar M (2013) Carbimazole is an inhibitor of protein synthesis and protects from neuronal hypoxic damage in vitro. J Pharmacol Exp Ther 347(3):781–793. https://doi.org/10.1124/jpet.113.205989
Li J, Ma L (2020) MiR-142-3p attenuates oxygen glucose deprivation/reoxygenation-induced injury by targeting FBXO3 in human neuroblastoma SH-SY5Y cells. World Neurosurg 136:e149–e157. https://doi.org/10.1016/j.wneu.2019.12.064
Li SH, Chen L, Pang XM, Su SY, Zhou X, Chen CY, Huang LG, Li JP, Liu JL (2017) Decreased miR-146a expression in acute ischemic stroke directly targets the Fbxl10 mRNA and is involved in modulating apoptosis. Neurochem Int 107:156–167. https://doi.org/10.1016/j.neuint.2017.01.011
Li J, Gu Z, Liu Y, Wang Y, Zhao M (2019a) Astilbin attenuates cerebral ischemia/reperfusion injury by inhibiting the TLR4/MyD88/NF-kappaB pathway. Toxicol Res (camb) 8(6):1002–1008. https://doi.org/10.1039/c9tx00222g
Li TF, Ma J, Han XW, Jia YX, Yuan HF, Shui SF, Guo D, Yan L (2019b) Chrysin ameliorates cerebral ischemia/reperfusion (I/R) injury in rats by regulating the PI3K/Akt/mTOR pathway. Neurochem Int 129:104496. https://doi.org/10.1016/j.neuint.2019.104496
Li WH, Yang YL, Cheng X, Liu M, Zhang SS, Wang YH, Du GH (2020a) Baicalein attenuates caspase-independent cells death via inhibiting PARP-1 activation and AIF nuclear translocation in cerebral ischemia/reperfusion rats. Apoptosis 25(5–6):354–369. https://doi.org/10.1007/s10495-020-01600-w
Li Y, Wang J, Chen S, Wu P, Xu S, Wang C, Shi H, Bihl J (2020b) miR-137 boosts the neuroprotective effect of endothelial progenitor cell-derived exosomes in oxyhemoglobin-treated SH-SY5Y cells partially via COX2/PGE2 pathway. Stem Cell Res Ther 11(1):330. https://doi.org/10.1186/s13287-020-01836-y
Lin D, Li G, Zuo Z (2011) Volatile anesthetic post-treatment induces protection via inhibition of glycogen synthase kinase 3beta in human neuron-like cells. Neuroscience 179:73–79. https://doi.org/10.1016/j.neuroscience.2011.01.055
Lin CH, Nicol CJB, Cheng YC, Yen C, Wang YS, Chiang MC (2020) Neuroprotective effects of resveratrol against oxygen glucose deprivation induced mitochondrial dysfunction by activation of AMPK in SH-SY5Y cells with 3D gelatin scaffold. Brain Res 1726:146492. https://doi.org/10.1016/j.brainres.2019.146492
Lin-Holderer J, Li L, Gruneberg D, Marti HH, Kunze R (2016) Fumaric acid esters promote neuronal survival upon ischemic stress through activation of the Nrf2 but not HIF-1 signaling pathway. Neuropharmacology 105:228–240. https://doi.org/10.1016/j.neuropharm.2016.01.023
Liu WF, Ou Y, Xu J, Zhang Z-J, Zhang G-X, Sun Y-W, Li S, Jian J (2016) Neuroprotective effect of apocynin nitrone in oxygen glucose deprivation-treated SH-SY5Y cells and rats with ischemic stroke. Trop J Pharm Res 15(8):1681–1689. https://doi.org/10.4314/tjpr.v15i8.13
Liu Y, Lu Z, Cui M, Yang Q, Tang Y, Dong Q (2016) Tissue kallikrein protects SH-SY5Y neuronal cells against oxygen and glucose deprivation-induced injury through bradykinin B2 receptor-dependent regulation of autophagy induction. J Neurochem 139(2):208–220. https://doi.org/10.1111/jnc.13690
Liu Y, Eaton ED, Wills TE, McCann SK, Antonic A, Howells DW (2018) Human ischaemic cascade studies using SH-SY5Y cells: a systematic review and meta-analysis. Transl Stroke Res 9(6):564–574. https://doi.org/10.1007/s12975-018-0620-4
Liu JY, Geng T, Duan K, Gao X, Huang CJ, Wang JJ, Huang WZ, Huang LS, Wang ZZ, Xiao W (2019a) Cellular pharmacokinetics and pharmacodynamics mechanisms of ginkgo diterpene lactone and its modulation of P-glycoprotein expression in human SH-SY5Y cells. Biomed Chromatogr 33(12):e4692. https://doi.org/10.1002/bmc.4692
Liu Q, Jin Z, Xu Z, Yang H, Li L, Li G, Li F, Gu S, Zong S, Zhou J, Cao L, Wang Z, Xiao W (2019b) Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones 24(2):441–452. https://doi.org/10.1007/s12192-019-00977-1
Liu ZD, Wang Q, Pan DQ, Meng FQ, Li JT, Wang YH (2020) MicroRNA-130b inhibits cerebral ischemia/reperfusion induced cell apoptosis via regulation of IRF1. Eur Rev Med Pharmacol Sci 24(23):12334–12341. https://doi.org/10.26355/eurrev_202012_24027
Liu N, Peng A, Sun H, Zhuang Y, Yu M, Wang Q, Wang J (2021) LncRNA AC1360072 alleviates cerebral ischemic-reperfusion injury by suppressing autophagy. Aging (albany NY) 13(15):19587–19597. https://doi.org/10.18632/aging.203369
Lu T, Zou Y, Zhou X, Peng W, Hu Z (2019) The mechanism on phosphorylation of Hsp20Ser16 inhibit GA stress and ER stress during OGD/R. PLoS ONE 14(3):e0213410. https://doi.org/10.1371/journal.pone.0213410
Luan H, Kan Z, Xu Y, Lv C, Jiang W (2013) Rosmarinic acid protects against experimental diabetes with cerebral ischemia: relation to inflammation response. J Neuroinflamm 10:28. https://doi.org/10.1186/1742-2094-10-28
Marmol F, Sanchez J, Martinez-Pinteno A (2021) Effects of uric acid on oxidative and nitrosative stress and other related parameters in SH-SY5Y human neuroblastoma cells. Prostaglandins Leukot Essent Fatty Acids 165:102237. https://doi.org/10.1016/j.plefa.2020.102237
Marutani E, Kosugi S, Tokuda K, Khatri A, Nguyen R, Atochin DN, Kida K, Van Leyen K, Arai K, Ichinose F (2012) A novel hydrogen sulfide-releasing N-methyl-d-aspartate receptor antagonist prevents ischemic neuronal death. J Biol Chem 287(38):32124–32135. https://doi.org/10.1074/jbc.M112.374124
Mattson MP, Lovell MA, Furukawa K, Markesbery WR (1995) Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J Neurochem 65(4):1740–1751. https://doi.org/10.1046/j.1471-4159.1995.65041740.x
McCune CD, Chan SJ, Beio ML, Shen W, Chung WJ, Szczesniak LM, Chai C, Koh SQ, Wong PT, Berkowitz DB (2016) “Zipped synthesis” by cross-metathesis provides a cystathionine beta-synthase inhibitor that attenuates cellular H2S levels and reduces neuronal infarction in a rat ischemic stroke model. ACS Cent Sci 2(4):242–252. https://doi.org/10.1021/acscentsci.6b00019
Meng Q, Yang P, Lu Y (2021) MicroRNA-410 serves as a candidate biomarker in hypoxic-ischemic encephalopathy newborns and provides neuroprotection in oxygen-glucose deprivation-injured PC12 and SH-SY5Y cells. Brain Behav 11(8):e2293. https://doi.org/10.1002/brb3.2293
Mestas J, Hughes CC (2004) Of mice and not men: differences between mouse and human immunology. J Immunol 172(5):2731–2738. https://doi.org/10.4049/jimmunol.172.5.2731
Mossink B, van Rhijn JR, Wang S, Linda K, Vitale MR, Zoller JEM, van Hugte EJH, Bak J, Verboven AHA, Selten M, Negwer M, Latour BL, van der Werf I, Keller JM, Klein Gunnewiek TM, Schoenmaker C, Oudakker A, Anania A, Jansen S, Nadif Kasri N (2021a) Cadherin-13 is a critical regulator of GABAergic modulation in human stem-cell-derived neuronal networks. Mol Psychiatry. https://doi.org/10.1038/s41380-021-01117-x
Mossink B, Verboven AHA, van Hugte EJH, Klein Gunnewiek TM, Parodi G, Linda K, Schoenmaker C, Kleefstra T, Kozicz T, van Bokhoven H, Schubert D, Nadif Kasri N, Frega M (2021b) Human neuronal networks on micro-electrode arrays are a highly robust tool to study disease-specific genotype-phenotype correlations in vitro. Stem Cell Rep 16(9):2182–2196. https://doi.org/10.1016/j.stemcr.2021.07.001
Mytilineou C, Leonardi EK, Radcliffe P, Heinonen EH, Han SK, Werner P, Cohen G, Olanow CW (1998) Deprenyl and desmethylselegiline protect mesencephalic neurons from toxicity induced by glutathione depletion. J Pharmacol Exp Ther 284(2):700–706
Nampoothiri SS, Rajanikant GK (2019) miR-9 upregulation integrates post-ischemic neuronal survival and regeneration in vitro. Cell Mol Neurobiol 39(2):223–240. https://doi.org/10.1007/s10571-018-0642-1
Nicholls DG, Ward MW (2000) Mitochondrial membrane potential and neuronal glutamate excitotoxicity: mortality and millivolts. Trends Neurosci 23(4):166–174. https://doi.org/10.1016/s0166-2236(99)01534-9
Niu G, Zhu D, Zhang X, Wang J, Zhao Y, Wang X (2018) Role of hypoxia-inducible factors 1alpha (HIF1alpha) in SH-SY5Y cell autophagy induced by oxygen-glucose deprivation. Med Sci Monit 24:2758–2766. https://doi.org/10.12659/MSM.905140
Olechnowicz SW, Fedele AO, Peet DJ (2012) Hypoxic induction of the regulator of G-protein signalling 4 gene is mediated by the hypoxia-inducible factor pathway. PLoS ONE 7(9):e44564. https://doi.org/10.1371/journal.pone.0044564
Pan H, Zhao F, Yang Y, Chang N (2020) Overexpression of long non-coding RNA SNHG16 against cerebral ischemia-reperfusion injury through miR-106b-5p/LIMK1 axis. Life Sci 254:117778. https://doi.org/10.1016/j.lfs.2020.117778
Park SY, Chae SY, Park JO, Lee KJ, Park G (2017) Kalopanacis cortex extract-capped gold nanoparticles activate NRF2 signaling and ameliorate damage in human neuronal SH-SY5Y cells exposed to oxygen-glucose deprivation and reoxygenation. Int J Nanomed 12:4563–4578. https://doi.org/10.2147/IJN.S138178
Pedersen ED, Aass HC, Rootwelt T, Fung M, Lambris JD, Mollnes TE (2007a) CD59 efficiently protects human NT2-N neurons against complement-mediated damage. Scand J Immunol 66(2–3):345–351. https://doi.org/10.1111/j.1365-3083.2007.01959.x
Pedersen ED, Froyland E, Kvissel AK, Pharo AM, Skalhegg BS, Rootwelt T, Mollnes TE (2007b) Expression of complement regulators and receptors on human NT2-N neurons—effect of hypoxia and reoxygenation. Mol Immunol 44(9):2459–2468. https://doi.org/10.1016/j.molimm.2006.10.022
Ping F, Zhang C, Wang X, Wang Y, Zhou D, Hu J, Chen Y, Ling J, Zhou J (2021) Cx32 inhibits the autophagic effect of Nur77 in SH-SY5Y cells and rat brain with ischemic stroke. Aging (albany NY) 13(18):22188–22207. https://doi.org/10.18632/aging.203526
Pires Monteiro S, Voogd E, Muzzi L, De Vecchis G, Mossink B, Levers M, Hassink G, van Putten M, le Feber J, Hofmeijer J, Frega M (2021) Neuroprotective effect of hypoxic preconditioning and neuronal activation in a human model of the ischemic penumbra. J Neural Eng. https://doi.org/10.1088/1741-2552/abe68a
Rosenthal LM, Tong G, Walker C, Wowro SJ, Krech J, Pfitzer C, Justus G, Berger F, Schmitt KRL (2017) Neuroprotection via RNA-binding protein RBM3 expression is regulated by hypothermia but not by hypoxia in human SK-N-SH neurons. Hypoxia (auckl) 5:33–43. https://doi.org/10.2147/HP.S132462
Roy K, Maji D, Deb I (2021) Increase of Cry 1 expression is a common phenomenon of the disturbed circadian clock in ischemic stroke and opioid addiction. Biochem Biophys Res Commun 558:8–13. https://doi.org/10.1016/j.bbrc.2021.04.053
Ruan ZF, Xie M, Gui SJ, Lan F, Wan J, Li Y (2020) MiR-370 accelerated cerebral ischemia reperfusion injury via targeting SIRT6 and regulating Nrf2/ARE signal pathway. Kaohsiung J Med Sci 36(9):741–749. https://doi.org/10.1002/kjm2.12219
Ruan L, Li G, Zhao W, Meng H, Zheng Q, Wang J (2021) Activation of adenosine A1 receptor in ischemic stroke: neuroprotection by tetrahydroxy stilbene glycoside as an agonist. Antioxidants (basel) 10(7):1112. https://doi.org/10.3390/antiox10071112
Shan W, Ge H, Chen B, Huang L, Zhu S, Zhou Y (2022) Upregulation of miR-499a-5p decreases cerebral ischemia/reperfusion injury by targeting PDCD4. Cell Mol Neurobiol 42(7):2157–2170. https://doi.org/10.1007/s10571-021-01085-4
Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J (2012) Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci Ther 18(3):250–260. https://doi.org/10.1111/j.1755-5949.2012.00295.x
Shi X, Yu W, Yang T, Liu W, Zhao Y, Sun Y, Chai L, Gao Y, Dong B, Zhu L (2016) Panax notoginseng saponins provide neuroprotection by regulating NgR1/RhoA/ROCK2 pathway expression, in vitro and in vivo. J Ethnopharmacol 190:301–312. https://doi.org/10.1016/j.jep.2016.06.017
Shi YS, Zhang Y, Liu B, Li CB, Wu J, Li Y (2019) Nomilin protects against cerebral ischemia-reperfusion induced neurological deficits and blood-brain barrier disruption via the Nrf2 pathway. Food Funct 10(9):5323–5332. https://doi.org/10.1039/c9fo01481k
Shi Y, Li K, Xu K, Liu QH (2020) MiR-155–5p accelerates cerebral ischemia-reperfusion injury via targeting DUSP14 by regulating NF-kappaB and MAPKs signaling pathways. Eur Rev Med Pharmacol Sci 24(3):1408–1419. https://doi.org/10.26355/eurrev_202002_20198
Shi Y, Yi Z, Zhao P, Xu Y, Pan P (2021) MicroRNA-532–5p protects against cerebral ischemia-reperfusion injury by directly targeting CXCL1. Aging (albany NY) 13(8):11528–11541. https://doi.org/10.18632/aging.202846
Shin TH, Phukan G, Shim JS, Nguyen DT, Kim Y, Oh-Lee JD, Lee HS, Paik MJ, Lee G (2016) Restoration of polyamine metabolic patterns in in vivo and in vitro model of ischemic stroke following human mesenchymal stem cell treatment. Stem Cells Int 2016:4612531. https://doi.org/10.1155/2016/4612531
Shipley MM, Mangold CA, Szpara ML (2016) Differentiation of the SH-SY5Y human neuroblastoma cell line. J vis Exp. https://doi.org/10.3791/53193
Sinoy S, Fayaz SM, Charles KD, Suvanish VK, Kapfhammer JP, Rajanikant GK (2017) Amikacin inhibits miR-497 maturation and exerts post-ischemic neuroprotection. Mol Neurobiol 54(5):3683–3694. https://doi.org/10.1007/s12035-016-9940-0
Soh H, Wasa M, Fukuzawa M (2007) Hypoxia upregulates amino acid transport in a human neuroblastoma cell line. J Pediatr Surg 42(4):608–612. https://doi.org/10.1016/j.jpedsurg.2006.12.010
Song J, Zhang W, Wang J, Yang H, Zhou Q, Wang H, Li L, Du G (2019) Inhibition of FOXO3a/BIM signaling pathway contributes to the protective effect of salvianolic acid A against cerebral ischemia/reperfusion injury. Acta Pharm Sin B 9(3):505–515. https://doi.org/10.1016/j.apsb.2019.01.010
Sriwastva MK, Kunjunni R, Andrabi M, Prasad K, Saxena R, Subbiah V (2020) Neuroprotective effects of activated protein C involve the PARP/AIF pathway against oxygen-glucose deprivation in SH-SY5Y cells. Brain Sci 10(12):959. https://doi.org/10.3390/brainsci10120959
Tajes M, Ill-Raga G, Palomer E, Ramos-Fernandez E, Guix FX, Bosch-Morato M, Guivernau B, Jimenez-Conde J, Ois A, Perez-Asensio F, Reyes-Navarro M, Caballo C, Galan AM, Alameda F, Escolar G, Opazo C, Planas A, Roquer J, Valverde MA, Munoz FJ (2013) Nitro-oxidative stress after neuronal ischemia induces protein nitrotyrosination and cell death. Oxid Med Cell Longev 2013:826143. https://doi.org/10.1155/2013/826143
Tan Y, Zhou F, Yang D, Zhang X, Zeng M, Wan L (2021) MicroRNA-126a-5p exerts neuroprotective effects on ischemic stroke via targeting NADPH oxidase 2. Neuropsychiatr Dis Treat 17:2089–2103. https://doi.org/10.2147/NDT.S293611
Tang SC, Yeh SJ, Li YI, Wang YC, Baik SH, Santro T, Widiapradja A, Manzanero S, Sobey CG, Jo DG, Arumugam TV, Jeng JS (2013) Evidence for a detrimental role of TLR8 in ischemic stroke. Exp Neurol 250:341–347. https://doi.org/10.1016/j.expneurol.2013.10.012
Taoufik E, Probert L (2008) Ischemic neuronal damage. Curr Pharm Des 14(33):3565–3573. https://doi.org/10.2174/138161208786848748
Tian C, Li Z, Zhang L, Dai D, Huang Q, Liu J, Hong B (2020) lncRNA NR_120420 promotes SH-SY5Y cells apoptosis by regulating NF-kappaB after oxygen and glucose deprivation. Gene 728:144285. https://doi.org/10.1016/j.gene.2019.144285
Tosetti P, Taglietti V, Toselli M (1998) Functional changes in potassium conductances of the human neuroblastoma cell line SH-SY5Y during in vitro differentiation. J Neurophysiol 79(2):648–658. https://doi.org/10.1152/jn.1998.79.2.648
Trotman-Lucas M, Gibson CL (2021) A review of experimental models of focal cerebral ischemia focusing on the middle cerebralartery occlusion model [version 2; peer review: 2 approved]. F1000Res 10:242. https://doi.org/10.12688/f1000research.51752.2
Wang Y, Xu M (2021) miR-380-5p facilitates NRF2 and attenuates cerebral ischemia/reperfusion injury-induced neuronal cell death by directly targeting BACH1. Transl Neurosci 12(1):210–217. https://doi.org/10.1515/tnsci-2020-0172
Wang N, Wu L, Cao Y, Wang Y, Zhang Y (2013) The protective activity of imperatorin in cultured neural cells exposed to hypoxia re-oxygenation injury via anti-apoptosis. Fitoterapia 90:38–43. https://doi.org/10.1016/j.fitote.2013.07.007
Wang J, Cao B, Han D, Sun M, Feng J (2017) Long non-coding RNA H19 induces cerebral ischemia reperfusion injury via activation of autophagy. Aging Dis 8(1):71–84. https://doi.org/10.14336/AD.2016.0530
Wang HF, Wang ZQ, Ding Y, Piao MH, Feng CS, Chi GF, Luo YN, Ge PF (2018a) Endoplasmic reticulum stress regulates oxygen-glucose deprivation-induced parthanatos in human SH-SY5Y cells via improvement of intracellular ROS. CNS Neurosci Ther 24(1):29–38. https://doi.org/10.1111/cns.12771
Wang M, Jiang YM, Xia LY, Wang Y, Li WY, Jin T (2018b) LncRNA NKILA upregulation mediates oxygen glucose deprivation/re-oxygenation-induced neuronal cell death by inhibiting NF-kappaB signaling. Biochem Biophys Res Commun 503(4):2524–2530. https://doi.org/10.1016/j.bbrc.2018.07.010
Wang R, Bao H, Zhang S, Li R, Chen L, Zhu Y (2018c) miR-186-5p promotes apoptosis by targeting IGF-1 in SH-SY5Y OGD/R model. Int J Biol Sci 14(13):1791–1799. https://doi.org/10.7150/ijbs.25352
Wang H, Liao S, Li H, Chen Y, Yu J (2019a) Long non-coding RNA TUG1 sponges Mir-145a-5p to regulate microglial polarization after oxygen-glucose deprivation. Front Mol Neurosci 12:215. https://doi.org/10.3389/fnmol.2019.00215
Wang J, Wang A, He H, She X, He Y, Li S, Liu L, Luo T, Huang N, Luo H, Zou K (2019b) Trametenolic acid B protects against cerebral ischemia and reperfusion injury through modulation of microRNA-10a and PI3K/Akt/mTOR signaling pathways. Biomed Pharmacother 112:108692. https://doi.org/10.1016/j.biopha.2019.108692
Wang T, Zhu L, Liu H, Yu G, Guo Y (2019c) Picroside II protects SH-SY5Y cells from autophagy and apoptosis following oxygen glucose deprivation/reoxygen injury by inhibiting JNK signal pathway. Anat Rec (hoboken) 302(12):2245–2254. https://doi.org/10.1002/ar.24214
Wang J, Chen J, Chen J, Liu X, Yang H, Liu J, He A, Gao X, Xin Y (2020a) KIF2 mediates the neuroprotection in cerebral ischaemia injury by affecting NF-kappaB pathway. Clin Exp Pharmacol Physiol 47(2):274–280. https://doi.org/10.1111/1440-1681.13175
Wang ZQ, Li K, Huang J, Huo TT, Lv PY (2020b) MicroRNA Let-7i is a promising serum biomarker for post-stroke cognitive impairment and alleviated OGD-induced cell damage in vitro by regulating Bcl-2. Front Neurosci 14:215. https://doi.org/10.3389/fnins.2020.00215
Wang D, Wang Y, Shan M, Chen J, Wang H, Sun B, Jin C, Li X, Yin Y, Song C, Xiao C, Li J, Wang T, Cai X (2021a) Apelin receptor homodimer inhibits apoptosis in vascular dementia. Exp Cell Res 407(1):112739. https://doi.org/10.1016/j.yexcr.2021.112739
Wang DW, Lou XQ, Liu ZL, Zhang N, Pang L (2021b) LncRNA SNHG1 protects SH-SY5Y cells from hypoxic injury through miR-140-5p/Bcl-XL axis. Int J Neurosci 131(4):336–345. https://doi.org/10.1080/00207454.2020.1744594
Wen Y, Zhang X, Liu X, Huo Y, Gao Y, Yang Y (2020) Suppression of lncRNA SNHG15 protects against cerebral ischemia-reperfusion injury by targeting miR-183–5p/FOXO1 axis. Am J Transl Res 12(10):6250–6263
Wu L, Jiang C, Kang Y, Dai Y, Fang W, Huang P (2020a) Curcumin exerts protective effects against hypoxiareoxygenation injury via the enhancement of apurinic/apyrimidinic endonuclease 1 in SHSY5Y cells: involvement of the PI3K/AKT pathway. Int J Mol Med 45(4):993–1004. https://doi.org/10.3892/ijmm.2020.4483
Wu S, Yang T, Cen K, Zou Y, Shi X, Zhou D, Gao Y, Chai L, Zhao Y, Sun Y, Zhu L (2020b) In vitro evaluation of the neuroprotective effect of panax notoginseng saponins by activating the EGFR/PI3K/AKT pathway. Evid Based Complement Alternat Med 2020:1403572. https://doi.org/10.1155/2020/1403572
Xing C, Yan G, Liu Q (2021) Inhibition of GPR4 attenuates SH-SY5Y cell injury in cerebral ischemia/reperfusion via anti-apoptotic pathways. Acta Biochim Pol 68(2):181–186. https://doi.org/10.18388/abp.2020_5497
Xu S, Li Y, Chen JP, Li DZ, Jiang Q, Wu T, Zhou XZ (2020a) Oxygen glucose deprivation/re-oxygenation-induced neuronal cell death is associated with Lnc-D63785 m6A methylation and miR-422a accumulation. Cell Death Dis 11(9):816. https://doi.org/10.1038/s41419-020-03021-8
Xu Z, Liu W, Huang H (2020b) Astragaloside IV alleviates cerebral ischemia-reperfusion injury by activating the janus kinase 2 and signal transducer and activator of transcription 3 signaling pathway. Pharmacology 105(3–4):181–189. https://doi.org/10.1159/000503361
Yan XL, Liu DH, Zhang GL, Hu SQ, Chen YG, Xu T (2015) S-Nitrosylation of proline-rich tyrosine kinase 2 involves its activation induced by oxygen-glucose deprivation. Neurosci Lett 597:90–96. https://doi.org/10.1016/j.neulet.2015.04.043
Yan Y, Chen L, Zhou J, Xie L (2020) SNHG12 inhibits oxygenglucose deprivationinduced neuronal apoptosis via the miR181a5p/NEGR1 axis. Mol Med Rep 22(5):3886–3894. https://doi.org/10.3892/mmr.2020.11459
Yanagita T, Manabe T, Okuda H, Matsuzaki S, Bando Y, Katayama T, Tohyama M (2005) Possible involvement of the expression and phosphorylation of N-Myc in the induction of HMGA1a by hypoxia in the human neuroblastoma cell line. Neurosci Lett 374(1):47–52. https://doi.org/10.1016/j.neulet.2004.10.039
Yang T, Sun S, Wang T, Tong X, Bi J, Wang Y, Sun Z (2014) Piperlonguminine is neuroprotective in experimental rat stroke. Int Immunopharmacol 23(2):447–451. https://doi.org/10.1016/j.intimp.2014.09.016
Yang H, Zhang Y, Chen H, Zhu Y, Li Y, Ouyang F, Chu L, Liu D (2021a) Mir-184 contributes to brain injury through targeting PPAP2B following ischemic stroke in male rats. Front Mol Neurosci 14:613887. https://doi.org/10.3389/fnmol.2021.613887
Yang T, Wang Q, Qu Y, Liu Y, Feng C, Wang Y, Sun W, Sun Z, Zhu Y (2021b) Punicalin alleviates OGD/R-triggered cell injury via TGF-beta-mediated oxidative stress and cell cycle in neuroblastoma cells SH-SY5Y. Evid Based Complement Alternat Med 2021:6671282. https://doi.org/10.1155/2021/6671282
Yao X, Yao R, Yi J, Huang F (2019) Upregulation of miR-496 decreases cerebral ischemia/reperfusion injury by negatively regulating BCL2L14. Neurosci Lett 696:197–205. https://doi.org/10.1016/j.neulet.2018.12.039
Yi Z, Shi Y, Zhao P, Xu Y, Pan P (2020) Overexpression of miR-217-5p protects against oxygen-glucose deprivation/reperfusion-induced neuronal injury via inhibition of PTEN. Hum Cell 33(4):1026–1035. https://doi.org/10.1007/s13577-020-00396-w
Yin M, Chen WP, Yin XP, Tu JL, Hu N, Li ZY (2021) LncRNA TUG1 demethylated by TET2 promotes NLRP3 expression, contributes to cerebral ischemia/reperfusion inflammatory injury. ASN Neuro 13:17590914211003248. https://doi.org/10.1177/17590914211003247
Yoon S-B, Lee G, Park SB, Cho H, Lee J-O, Koh B (2020) Properties of differentiated SH-SY5Y grown on carbon-based materials. RSC Adv 10(33):19382–19389. https://doi.org/10.1039/D0RA03383A
Zappala A, Vicario N, Calabrese G, Turnaturi R, Pasquinucci L, Montenegro L, Spadaro A, Parenti R, Parenti C (2021) Neuroprotective effects of Rosmarinus officinalis L. extract in oxygen glucose deprivation (OGD)-injured human neural-like cells. Nat Prod Res 35(4):669–675. https://doi.org/10.1080/14786419.2019.1587428
Zeng Z, Zhang Y, Liang X, Wang F, Zhao J, Xu Z, Liu X, Liu X (2019) Qingnao dripping pills mediate immune-inflammatory response and MAPK signaling pathway after acute ischemic stroke in rats. J Pharmacol Sci 139(3):143–150. https://doi.org/10.1016/j.jphs.2018.12.009
Zhang D, Zhao N, Ma B, Wang Y, Zhang G, Yan X, Hu S, Xu T (2016a) Procaspase-9 induces its cleavage by transnitrosylating XIAP via the Thioredoxin system during cerebral ischemia-reperfusion in rats. Sci Rep 6:24203. https://doi.org/10.1038/srep24203
Zhang JF, Shi LL, Zhang L, Zhao ZH, Liang F, Xu X, Zhao LY, Yang PB, Zhang JS, Tian YF (2016b) MicroRNA-25 negatively regulates cerebral ischemia/reperfusion injury-induced cell apoptosis through Fas/FasL pathway. J Mol Neurosci 58(4):507–516. https://doi.org/10.1007/s12031-016-0712-0
Zhang YT, Li FM, Guo YZ, Jiang LR, Ma J, Ke Y, Qian ZM (2016c) (Z)-ligustilide increases ferroportin1 expression and ferritin content in ischemic SH-SY5Y cells. Eur J Pharmacol 792:48–53. https://doi.org/10.1016/j.ejphar.2016.10.029
Zhang H, Zhou J, Zhang M, Yi Y, He B (2019a) Upregulation of miR-376c-3p alleviates oxygen-glucose deprivation-induced cell injury by targeting ING5. Cell Mol Biol Lett 24:67. https://doi.org/10.1186/s11658-019-0189-2
Zhang X, Fu C, Chen B, Xu Z, Zeng Z, He L, Lu Y, Chen Z, Liu X (2019b) Autophagy induced by oxygen-glucose deprivation mediates the injury to the neurovascular unit. Med Sci Monit 25:1373–1382. https://doi.org/10.12659/MSM.915123
Zhang ZB, Tan YX, Zhao Q, Xiong LL, Liu J, Xu FF, Xu Y, Bobrovskaya L, Zhou XF, Wang TH (2019c) miRNA-7a-2-3p inhibits neuronal apoptosis in oxygen-glucose deprivation (OGD) model. Front Neurosci 13:16. https://doi.org/10.3389/fnins.2019.00016
Zhang L, Liu C, Huang C, Xu X, Teng J (2020a) miR-155 knockdown protects against cerebral ischemia and reperfusion injury by targeting MafB. Biomed Res Int 2020:6458204. https://doi.org/10.1155/2020/6458204
Zhang ZH, Wang YR, Li F, Liu XL, Zhang H, Zhu ZZ, Huang H, Xu XH (2020b) Circ-camk4 involved in cerebral ischemia/reperfusion induced neuronal injury. Sci Rep 10(1):7012. https://doi.org/10.1038/s41598-020-63686-1
Zhao J, Wang B (2020) MiR-7-5p enhances cerebral ischemia-reperfusion injury by degrading sirt1 mRNA. J Cardiovasc Pharmacol 76(2):227–236. https://doi.org/10.1097/FJC.0000000000000852
Zhao LP, Ji C, Lu PH, Li C, Xu B, Gao H (2013) Oxygen glucose deprivation (OGD)/re-oxygenation-induced in vitro neuronal cell death involves mitochondrial cyclophilin-D/P53 signaling axis. Neurochem Res 38(4):705–713. https://doi.org/10.1007/s11064-013-0968-5
Zhi SM, Fang GX, Xie XM, Liu LH, Yan J, Liu DB, Yu HY (2020) Melatonin reduces OGD/R-induced neuron injury by regulating redox/inflammation/apoptosis signaling. Eur Rev Med Pharmacol Sci 24(3):1524–1536. https://doi.org/10.26355/eurrev_202002_20211
Zhou M, Yang WL, Ji Y, Qiang X, Wang P (2014) Cold-inducible RNA-binding protein mediates neuroinflammation in cerebral ischemia. Biochim Biophys Acta 1840(7):2253–2261. https://doi.org/10.1016/j.bbagen.2014.02.027
Zhou X, Su S, Li S, Pang X, Chen C, Li J, Liu J (2016) MicroRNA-146a down-regulation correlates with neuroprotection and targets pro-apoptotic genes in cerebral ischemic injury in vitro. Brain Res 1648(Pt A):136–143. https://doi.org/10.1016/j.brainres.2016.07.034
Zhou Z, Xu H, Liu B, Dun L, Lu C, Cai Y, Wang H (2019) Suppression of lncRNA RMRP ameliorates oxygen-glucose deprivation/re-oxygenation-induced neural cells injury by inhibiting autophagy and PI3K/Akt/mTOR-mediated apoptosis. Biosci Rep. https://doi.org/10.1042/BSR20181367
Zuo ML, Wang AP, Song GL, Yang ZB (2020) miR-652 protects rats from cerebral ischemia/reperfusion oxidative stress injury by directly targeting NOX2. Biomed Pharmacother 124:109860. https://doi.org/10.1016/j.biopha.2020.109860
Acknowledgements
Graphical abstract was created with Biorender.com.
Funding
This research has been supported by an institutional research grant.
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Voogd, E.J.H.F., Frega, M. & Hofmeijer, J. Neuronal Responses to Ischemia: Scoping Review of Insights from Human-Derived In Vitro Models. Cell Mol Neurobiol 43, 3137–3160 (2023). https://doi.org/10.1007/s10571-023-01368-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-023-01368-y