Abstract
This review is on how current knowledge of brainstem control of gastric mechanical function unfolded over nearly four decades from the perspective of our research group. It describes data from a multitude of different types of studies involving retrograde neuronal tracing, microinjection of drugs, whole-cell recordings from rodent brain slices, receptive relaxation reflex, accommodation reflex, c-Fos experiments, immunohistochemical methods, electron microscopy, transgenic mice, optogenetics, and GABAergic signaling. Data obtained indicate the following: (1) nucleus tractus solitarius (NTS)—dorsal motor nucleus of the vagus (DMV) noradrenergic connection is required for reflex control of the fundus; (2) second-order nitrergic neurons in the NTS are also required for reflex control of the fundus; (3) a NTS GABAergic connection is required for reflex control of the antrum; (4) a single DMV efferent pathway is involved in brainstem control of gastric mechanical function under most experimental conditions excluding the accommodation reflex. Dual-vagal effectors controlling cholinergic and non-adrenergic and non-cholinergic (NANC) input to the stomach may be part of the circuitry of this reflex. (5) GABAergic signaling within the NTS via Sst-GABA interneurons determine the basal (resting) state of gastric tone and phasic contractions. (6) For the vagal–vagal reflex to become operational, an endogenous opioid in the NTS is released and the activity of Sst-GABA interneurons is suppressed. From the data, we suggest that the CNS has the capacity to provide region-specific control over the proximal (fundus) and distal (antrum) stomach through engaging phenotypically different efferent inputs to the DMV.

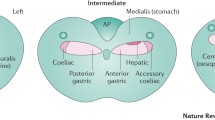
Adapted from Fig. 13 of Ferreira et al. (2002)


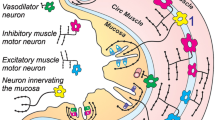
Adapted from Fig. 9 of Herman et al. (2009)

Similar content being viewed by others
References
Abrahamsson H, Jansson G (1969) Elicitation of reflex vagal relaxation of the stomach from pharynx and esophagus in the cat. Acta Physiol Scand 77(1–2):172–178
Andrews PL, Lawes IN (1985) Gastric tone modifies the responses to extrinsic neural stimuli in the anaesthetized ferret. J Physiol (Lond) 366(1):1–16
Andrews PR (1990) Central organization of the vagal drive to the nonadrenergic, noncholonergic neurones controlling gastric motility. Arch Int Pharmacodyn Ther 303:167–198
Anselmi L, Toti L, Bove C et al (2017) Vagally mediated effects of brain stem dopamine on gastric tone and phasic contractions of the rat. Am J Physiol-Gastrointest Liver Physiol 313(5):G434–G441
Bertolino M, Vicini S, Gillis R et al (1997) Presynaptic alpha2-adrenoceptors inhibit excitatory synaptic transmission in rat brain stem. Am J Physiol Gastrointest Liver Physiol 272(3):G654–G661. https://doi.org/10.1152/ajpgi.1997.272.3.G654
Bronstein DM, Schafer MK, Watson SJ et al (1992) Evidence that β-endorphin is synthesized in cells in the nucleus tractus solitarius: detection of POMC mRNA. Brain Res 587(2):269–275. https://doi.org/10.1016/0006-8993(92)91007-2
Buelke-Sam J, Holson JF, Bazare JJ et al (1978) Comparative stability of physiological parameters during sustained anesthesia in rats. Lab Anim Sci 28(2):157–162
Bülbül M, Sinen O (2018) Dual autonomic inhibitory action of central Apelin on gastric motor functions in rats. Auton Neurosci 212:17–22
Christie MJ, Connor M, Vaughan CW et al (2000) Cellular actions of opioids and other analgesics: implications for synergism in pain relief. Clin Exp Pharmacol Physiol 27(7):520–523. https://doi.org/10.1046/j.1440-1681.2000.03291.x
Colquhoun LM, Patrick JW (1997) Pharmacology of neuronal nicotinic acetylcholine receptor subtypes. Anon Adv Pharmacol 39:191–220
Cruz MT (2006) Characterization of DMV pathways controlling gastric motility in the rat. Ph.D., Georgetown University Medical Center, Washington, DC
Cruz MT, Murphy EC, Sahibzada N et al (2007) A reevaluation of the effects of stimulation of the dorsal motor nucleus of the vagus on gastric motility in the rat. Am J Physiol-Regul Integr Comp Physiol 292(1):R291–R307. https://doi.org/10.1152/ajpregu.00863.2005
Cruz MT, Dezfuli G, Murphy E et al (2019) GABAB receptor signaling in the dorsal motor nucleus of the vagus stimulates gastric motility via a cholinergic pathway. Front Neurosci 13:967
Davis SF, Derbenev AV, Williams KW et al (2004) Excitatory and inhibitory local circuit input to the rat dorsal motor nucleus of the vagus originating from the nucleus tractus solitarius. Brain Res 1017(1–2):208–217
Desai KM, Sessa WC, Vane JR (1991a) Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature 351(6326):477–479
Desai KM, Zembowicz A, Sessa WC et al (1991b) Nitroxergic nerves mediate vagally induced relaxation in the isolated stomach of the guinea pig. Proc Natl Acad Sci 88(24):11490–11494
Ellison DH, Velazquez H, Wright FS (1987) Thiazide-sensitive sodium chloride cotransport in early distal tubule. Am J Physiol-Renal Physiol 253(3):F546–F554. https://doi.org/10.1152/ajprenal.1987.253.3.F546
Fanselow EE, Connors BW (2010) The roles of somatostatin-expressing (GIN) and fast-spiking inhibitory interneurons in up-down states of mouse neocortex. J Neurophysiol 104(2):596–606. https://doi.org/10.1152/jn.00206.2010
Fanselow EE, Richardson KA, Connors BW (2008) Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol 100(5):2640–2652. https://doi.org/10.1152/jn.90691.2008
Faraday MM (2002) Rat sex and strain differences in responses to stress. Physiol Behav 75(4):507–522
Ferreira M, Ebert SN, Perry DC et al (2001) Evidence of a functional α7-neuronal nicotinic receptor subtype located on motoneurons of the dorsal motor nucleus of the vagus. J Pharmacol Exp Ther 296(2):260–269
Ferreira M, Sahibzada N, Shi M et al (2005) Hindbrain chemical mediators of reflex-induced inhibition of gastric tone produced by esophageal distension and intravenous nicotine. Am J Physiol-Regul Integr Comp Physiol 289(5):R1482–R1495. https://doi.org/10.1152/ajpregu.00003.2005
Ferreira M, Sahibzada N, Shi M et al (2002) CNS site of action and brainstem circuitry responsible for the intravenous effects of nicotine on gastric tone. J Neurosci 22(7):2764–2779
Ferreira M, Singh A, Dretchen KL et al (2000) Brainstem nicotinic receptor subtypes that influence intragastric and arterial blood pressures. J Pharmacol Exp Ther 294(1):230–238
Fong AY, Stornetta RL, Foley CM et al (2005) Immunohistochemical localization of GAD67-expressing neurons and processes in the rat brainstem: subregional distribution in the nucleus tractus solitarius. J Comp Neurol 493(2):274–290. https://doi.org/10.1002/cne.20758
Fukuda A, Minami T, Nabekura J et al (1987) The effects of noradrenaline on neurones in the rat dorsal motor nucleus of the vagus, in vitro. J Physiol (Lond) 393(1):213–231
Gao H, Glatzer NR, Williams KW et al (2009) Morphological and electrophysiological features of motor neurons and putative interneurons in the dorsal vagal complex of rats and mice. Brain Res 1291:40–52
Geeraerts B, Mimidis K, Van Oudenhove L et al (2008) Role of endogenous opioids in the control of gastric sensorimotor function. Neurogastroenterol Motil 20(10):1094–1102. https://doi.org/10.1111/j.1365-2982.2008.01144.x
Gentet LJ, Kremer Y, Taniguchi H et al (2012) Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci 15(4):607–612
Glatzer NR, Derbenev AV, Banfield BW et al (2007) Endomorphin-1 modulates intrinsic inhibition in the dorsal vagal complex. J Neurophysiol 98(3):1591–1599. https://doi.org/10.1152/jn.00336.2007
Glatzer NR, Smith BN (2005) Modulation of synaptic transmission in the rat nucleus of the solitary tract by endomorphin-1. J Neurophysiol 93(5):2530–2540. https://doi.org/10.1152/jn.00429.2004
Halabisky B, Shen F, Huguenard JR et al (2006) Electrophysiological classification of somatostatin-positive interneurons in mouse sensorimotor cortex. J Neurophysiol 96(2):834–845
Harrington AM, Hutson JM, Southwell BR (2007) Immunohistochemical localisation of cholinergic muscarinic receptor subtype 1 (M1r) in the guinea pig and human enteric nervous system. J Chem Neuroanat 33(4):193–201
Hennig GW, Spencer NJ (2018) Chapter 21—Physiology of gastric motility patterns. In: Said HM (ed) Physiology of the gastrointestinal tract, 6th edn. Academic Press, Burlingtonp, pp 469–484
Herman MA, Cruz MT, Sahibzada N et al (2009) GABA signaling in the nucleus tractus solitarius sets the level of activity in dorsal motor nucleus of the vagus cholinergic neurons in the vagovagal circuit. Am J Physiol-Gastrointest Liver Physiol 296(1):G101–G111. https://doi.org/10.1152/ajpgi.90504.2008
Herman MA, Niedringhaus M, Alayan A et al (2008) Characterization of noradrenergic transmission at the dorsal motor nucleus of the vagus involved in reflex control of fundus tone. Am J Physiol-Regul Integr Comp Physiol 294(3):R720–R729. https://doi.org/10.1152/ajpregu.00630.2007
Herman MA, Alayan A, Sahibzada N et al (2010) μ-Opioid receptor stimulation in the medial subnucleus of the tractus solitarius inhibits gastric tone and motility by reducing local GABA activity. Am J Physiol-Gastrointest Liver Physiol 299(2):G494–G506
Hermann GE, Alberto Travagli R, Rogers RC (2006) Esophageal-gastric relaxation reflex in rat: dual control of peripheral nitrergic and cholinergic transmission. Am J Physiol-Regul Integr Comp Physiol 290(6):R1570–R1576. https://doi.org/10.1152/ajpregu.00717.2005
Heyer EJ, Macdonald RL (1982) Barbiturate reduction of calcium-dependent action potentials: CORRELATION with anesthetic action. Brain Res 236(1):157–171. https://doi.org/10.1016/0006-8993(82)90042-7
Holmes GM, Browning KN, Babic T et al (2013) Vagal afferent fibres determine the oxytocin-induced modulation of gastric tone. J Physiol (Lond) 591(12):3081–3100
Hornby PJ, Rossiter CD, White RL et al (1990) Medullary raphe: a new site for vagally mediated stimulation of gastric motility in cats. Am J Physiol Gastrointest Liver Physiol 258(4):G637–G647. https://doi.org/10.1152/ajpgi.1990.258.4.G637
Hornby PJ (2001) II. Excitatory amino acid receptors in the brain-gut axis. Am J Physiol Gastrointest Liver Physiol 280(6):G1055–G1060. https://doi.org/10.1152/ajpgi.2001.280.6.G1055
Jackson DM, Richards IM (1977) The effects of pentobarbitone and chloralose anaesthesia on the vagal component of bronchoconstriction produced by histamine aerosol in the anaesthetized dog. Br J Pharmacol 61(2):251
Jiang Y, Browning KN, Toti L et al (2018) Vagally mediated gastric effects of brain stem α2-adrenoceptor activation in stressed rats. Am J Physiol-Gastrointest Liver Physiol 314(4):G504–G516. https://doi.org/10.1152/ajpgi.00382.2017
MorrisonHeymanasRichardson JLCAP et al (1951) The comparative action of certain ganglionic blocking agents. Arch Int Pharmacodyn Ther 86(2):203–213
Kishi T, Aschkenasi CJ, Lee CE et al (2003) Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457(3):213–235. https://doi.org/10.1002/cne.10454
Kobashi M, Koga T, Mizutani M et al (2002) Suppression of vagal motor activities evokes laryngeal afferent-mediated inhibition of gastric motility. Am J Physiol-Regul Integr Comp Physiol 282(3):R818–R827. https://doi.org/10.1152/ajpregu.00180.2001
Kobashi M, Mizutani M, Matsuo R (2000) Water stimulation of the posterior oral cavity induces inhibition of gastric motility. Am J Physiol-Regul Integr Comp Physiol 279(3):R778–R785. https://doi.org/10.1152/ajpregu.2000.279.3.R778
Koppanyi T, Linegar CR, Dille JM (1935) The peripheral action of barbiturates. Science 82(2123):232
Korner PI, Langsford G, Starr D et al (1968) The effects of chloralose-urethane and sodium pentobarbitone anaesthesia on the local and autonomic components of the circulatory response to arterial hypoxia. J Physiol (Lond) 199(2):283–302
Kortezova NI, Shikova LI, Milusheva EA et al (2004) Muscarinic modulation of nitrergic neurotransmission in guinea-pig gastric fundus. Neurogastroenterol Motil 16(2):155–165
Krowicki ZK, Sharkey KA, Serron SC et al (1997) Distribution of nitric oxide synthase in rat dorsal vagal complex and effects of microinjection of nitric oxide compounds upon gastric motor function. J Comp Neurol 377(1):49–69
Krowicki ZK, Sivarao DV, Abrahams TP et al (1999) Excitation of dorsal motor vagal neurons evokes non-nicotinic receptor-mediated gastric relaxation. J Auton Nerv Syst 77(2–3):83–89
Laiprasert JD, Hamlin RL, Heesch CM (2001) Afferent baroreceptor discharge in pregnant rats. Am J Physiol-Heart Circ Physiol 281(6):H2456–H2462
Laycock JF, Penn W, Shirley DG et al (1979) The role of vasopressin in blood pressure regulation immediately following acute haemorrhage in the rat. J Physiol (Lond) 296(1):267–275
Lewin AE, Vicini S, Richardson J et al (2016) Optogenetic and pharmacological evidence that somatostatin-GABA neurons are important regulators of parasympathetic outflow to the stomach. J Physiol 594(10):2661–2679. https://doi.org/10.1113/JP272069
Lewis MW, Hermann GE, Rogers RC et al (2002) In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J Physiol (Lond) 543(1):135–146. https://doi.org/10.1113/jphysiol.2002.019281
Lin L, Talman WT (2000) N-methyl-D-aspartate receptors on neurons that synthesize nitric oxide in rat nucleus tractus solitarii. Neuroscience 100(3):581–588
Liu H, Kishi T, Roseberry AG et al (2003) Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci 23(18):7143–7154
Ma S, Abboud FM, Felder RB (1995) Effects of L-arginine-derived nitric oxide synthesis on neuronal activity in nucleus tractus solitarius. Am J Physiol-Regul Integr Comp Physiol 268(2):R487–R491
Martin-Schild S, Gerall AA, Kastin AJ et al (1999) Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol 405(4):450–471. https://doi.org/10.1002/(SICI)1096-9861(19990322)405:43.0.CO;2-#
McCann MJ, Rogers RC (1992) Impact of antral mechanoreceptor activation on the vago-vagal reflex in the rat: functional zonation of responses. J Physiol (Lond) 453(1):401–411. https://doi.org/10.1113/jphysiol.1992.sp019235
McGehee DS, Role LW (1995) Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol 57(1):521–546. https://doi.org/10.1146/annurev.ph.57.030195.002513
Bülbül M, Sinen O, Gök M et al (2018) Apelin-13 inhibits gastric motility through vagal cholinergic pathway in rats. Am J Physiol-Gastrointest Liver Physiol 314(2):G201–G210. https://doi.org/10.1152/ajpgi.00223.2017
Millard P (2003) Anesthetic procedures. In: Aspinall V (ed) Clininical procedures in veterinary medicine, 2nd edn. Elsevier, Saunders, pp 121–155
Mountjoy KG, Mortrud MT, Low MJ et al (1994) Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 8(10):1298–1308
Murthy VS, Zagar ME, Vollmer RR et al (1982) Pentobarbital-induced changes in vagal tone and reflex vagal activity in rabbits. Eur J Pharmacol 84(1–2):41–50
Mutolo D, Bongianni F, Cinelli E et al (2008) Modulation of the cough reflex by antitussive agents within the caudal aspect of the nucleus tractus solitarii in the rabbit. Am J Physiol-Regul Integr Comp Physiol 295(1):R243–R251. https://doi.org/10.1152/ajpregu.00184.2008
Niedringhaus M, Jackson PG, Pearson R et al (2008) Brainstem sites controlling the lower esophageal sphincter and crural diaphragm in the ferret: a neuroanatomical study. Auton Neurosci 144(1–2):50–60
Norman WP, Pagani FD, Ormsbee HS III et al (1985) Use of horseradish peroxidase to identify hindbrain sites that influence gastric motility in the cat. Gastroenterology 88(3):701–705
O’dell TJ, Hawkins RD, Kandel ER et al (1991) Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci 88(24):11285–11289
Oliva AA, Jiang M, Lam T et al (2000) Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci 20(9):3354–3368
Oltra-Noguera D, Mangas-Sanjuan V, González-Álvarez I et al (2015) Drug gastrointestinal absorption in rat: strain and gender differences. Eur J Pharm Sci 78:198–203
Padilla SL, Reef D, Zeltser LM (2012) Defining POMC neurons using transgenic reagents: impact of transient Pomc expression in diverse immature neuronal populations. Endocrinology 153(3):1219–1231
Pagani FD, Norman WP, Kasbekar DK et al (1985) Localization of sites within dorsal motor nucleus of vagus that affect gastric motility. Am J Physiol-Gastrointest Liver Physiol 249(1):G73–G84
Pagani FD, Norman WP, Gillis RA (1988) Medullary parasympathetic projections innervate specific sites in the feline stomach. Gastroenterology 95(2):277–288
Paxinos G (1999) Chemoarchitectonic atlas of the rat forebrain. Academic, San Diego
Pearson RJ, Gatti PJ, Sahibzada N et al (2007) Ultrastructural evidence for selective noradrenergic innervation of CNS vagal projections to the fundus of the rat. Auton Neurosci 136(1):31–42. https://doi.org/10.1016/j.autneu.2007.03.003
Pearson RJ, Gatti PJ, Sahibzada N et al (2011) Ultrastructural evidence for selective GABAergic innervation of CNS vagal projections to the antrum of the rat. Auton Neurosci 160(1):21–26. https://doi.org/10.1016/j.autneu.2010.10.010
Pickel VM, Chan J, Park DH et al (1986) Ultrastructural localization of phenylethanolamine N-methyltransferase in sensory and motor nuclei of the vagus nerve. J Neurosci Res 15(4):439–455
Pierce TL, Wessendorf MW (2000) Immunocytochemical mapping of endomorphin-2-immunoreactivity in rat brain. J Chem Neuroanat 18(4):181–207. https://doi.org/10.1016/S0891-0618(00)00042-9
Pröve J, Ehrlein HJ (1982) Motor function of gastric antrum and pylorus for evacuation of low and high viscosity meals in dogs. Gut 23(2):150–156
Qualls-creekmore E, Tong M, Holmes GM (2010) Gastric emptying of enterally administered liquid meal in conscious rats and during sustained anaesthesia. Neurogastroenterol Motil 22(2):181–185
Richardson J, Cruz MT, Majumdar U et al (2013) Melanocortin signaling in the brainstem influences vagal outflow to the stomach. J Neurosci 33(33):13286–13299. https://doi.org/10.1523/JNEUROSCI.0780-13.2013
Rogers RC, Travagli RA, Hermann GE (2003) Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol-Regul Integr Comp Physiol 285(2):R479–R489
Rogers R, Hermann G (2012) Brainstem control of the gastric function. Physiol Gastrointest Tract 1:861–891. https://doi.org/10.1016/B978-0-12-382026-6.00031-2
Rossiter CD, Norman WP, Jain M et al (1990) Control of lower esophageal sphincter pressure by two sites in dorsal motor nucleus of the vagus. Am J Physiol-Gastrointest Liver Physiol 259(6):G899–G906. https://doi.org/10.1152/ajpgi.1990.259.6.G899
Royer S, Zemelman BV, Losonczy A et al (2012) Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci 15(5):769–775
Ruggiero DA, Mtui EP, Otake K et al (1996) Central and primary visceral afferents to nucleus tractus solitarii may generate nitric oxide as a membrane-permeant neuronal messenger. J Comp Neurol 364(1):51–67
Sababi M, Nylander O (1996) Comparative study of the effects of nitric oxide synthase and cyclo-oxygenase inhibition on duodenal functions in rats anaesthetized with inactin, urethane or a-chloralose. Acta Physiol Scand 158(1):45–52
Scanlin HL, Carroll EA, Jenkins VK et al (2008) Endomorphin-2 is released from newborn rat primary sensory neurons in a frequency- and calcium-dependent manner. Eur J Neurosci 27(10):2629–2642. https://doi.org/10.1111/j.1460-9568.2008.06238.x
Schulze-Delrieu K (1983) Volume accommodation by distension of gastric fundus (rabbit) and gastric corpus (cat). Dig Dis Sci 28(7):625–632
Shapiro RE, Miselis RR (1985) The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol 238(4):473–488. https://doi.org/10.1002/cne.902380411
Shi M, Jones AR, Niedringhaus MS et al (2003) Glucose acts in the CNS to regulate gastric motility during hypoglycemia. Am J Physiol-Regul Integr Comp Physiol 285(5):R1192–R1202. https://doi.org/10.1152/ajpregu.00179.2003
Shi M, Jones AR, Ferreira M Jr et al (2005) Glucose does not activate nonadrenergic, noncholinergic inhibitory neurons in the rat stomach. Am J Physiol-Regul Integr Comp Physiol 288(3):R742–R750
Shirley DG, Walter SJ (1995) A micropuncture study of the renal response to haemorrhage in rats: assessment of the role of vasopressin. Exp Physiol 80(4):619–630
Siaud P, Denoroy L, Assenmacher I et al (1989) Comparative immunocytochemical study of the catecholaminergic and peptidergic afferent innervation to the dorsal vagal complex in rat and guinea pig. J Comp Neurol 290(3):323–335. https://doi.org/10.1002/cne.902900302
Sivarao DV, Krowicki ZK, Hornby PJ (1998) Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil 10(4):305–313
Smith BN, Dou P, Barber WD et al (1998) Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol (Lond) 512(1):149–162
Spencer SE, Talman WT (1986) Modulation of gastric and arterial pressure by nucleus tractus solitarius in rat. Am J Physiol-Regul Integr Comp Physiol 250(6):R996–R1002. https://doi.org/10.1152/ajpregu.1986.250.6.R996
Tack J (2012) Chapter 34—Neurophysiologic mechanisms of gastric reservoir function. In: Johnson LR, Ghishan FK, Kaunitz JD et al (eds) Physiology of the gastrointestinal tract, 5th edn. Academic Press, Boston, pp 951–957
Takahashi T, Owyang C (1997) Characterization of vagal pathways mediating gastric accommodation reflex in rats. J Physiol (Lond) 504(Pt 2):479
Talman WT, Andreasen K, Calvin J et al (1991) Cholecystokinin in nucleus tractus solitarii modulates tonic and phasic gastric pressure. Am J Physiol-Regul Integr Comp Physiol 261(1):R217–R222. https://doi.org/10.1152/ajpregu.1991.261.1.R217
Talman WT, Perrone MH, Reis DJ (1980) Evidence for L-glutamate as the neurotransmitter of baroreceptor afferent nerve fibers. Science 209(4458):813–815
Tan AA, Quigley A, Smith DC et al (2009) Strain differences in response to traumatic brain injury in Long-Evans compared to Sprague-Dawley rats. J Neurotrauma 26(4):539–548
Taniguchi H, He M, Wu P et al (2011) A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71(6):995–1013. https://doi.org/10.1016/j.neuron.2011.07.026
Thek KR, Ong SJ, Carter DC et al (2019) Extensive inhibitory gating of viscerosensory signals by a sparse network of somatostatin neurons. J Neurosci 39(41):8038–8050
Torjman MC, Joseph JI, Munsick C, Morishita M, Grunwald Z (2005) Effects of Isoflurane on gastrointestinal motility after brief exposure in rats. Int J Pharm 294(1–2):65–71. https://doi.org/10.1016/j.ijpharm.2004.12.028
Travagli RA, Hermann GE, Browning KN et al (2003) Musings on the wanderer: what’s new in our understanding of vago-vagal reflexes?: III. Activity-dependent plasticity in vago-vagal reflexes controlling the stomach. Am J Physiol-Gastrointest Liver Physiol 284(2):G180
Travagli RA, Gillis RA, Rossiter CD et al (1991) Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol-Gastrointest Liver Physiol 260(3):G531–G536. https://doi.org/10.1152/ajpgi.1991.260.3.G531
Tucker BJ, Peterson OW, Ziegler MG et al (1982) Analysis of adrenergic effects of the anesthetics inactin and alpha-chloralose. Am J Physiol-Ren Physiol 243(3):F253–F259
Viard E, Sapru HN (2006) Endomorphin-2 in the medial NTS attenuates the responses to baroreflex activation. Brain Res 1073–1074:365–373. https://doi.org/10.1016/j.brainres.2005.12.102
Vitela M, Herrera-Rosales M, Haywood JR et al (2005) Baroreflex regulation of renal sympathetic nerve activity and heart rate in renal wrap hypertensive rats. Am J Physiol-Regul Integr Comp Physiol 288(4):R856–R862
Wang D, He X, Zhao Z et al (2015) Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front Neuroanat 9:40
Wang J, Irnaten M, Mendelowitz D (2001) Characteristics of spontaneous and evoked GABAergic synaptic currents in cardiac vagal neurons in rats. Brain Res 889(1):78–83. https://doi.org/10.1016/S0006-8993(00)03112-7
Wang M, Bradley RM (2010) Properties of GABAergic neurons in the rostral solitary tract nucleus in mice. J Neurophysiol 103(6):3205–3218
Wasserman AM, Ferreira M, Sahibzada N et al (2002) GABA-mediated neurotransmission in the ventrolateral NTS plays a role in respiratory regulation in the rat. Am J Physiol-Regul Integr Comp Physiol 283(6):R1423–R1441. https://doi.org/10.1152/ajpregu.00488.2001
Watson C (2014) Paxinos and Watson’s the rat brain in stereotaxic coordinates, 7th edn. Elsevier, Amsterdam
Willing AE, Berthoud H (1997) Gastric distension-induced c-Fos expression in catecholaminergic neurons of rat dorsal vagal complex. Am J Physiol-Regul Integr Comp Physiol 272(1):R59–R67
Willis A, Mihalevich M, Neff RA et al (1996) Three types of postsynaptic glutamatergic receptors are activated in DMNX neurons upon stimulation of NTS. Am J Physiol-Regul Integr Comp Physiol 271(6):R1614–R1619. https://doi.org/10.1152/ajpregu.1996.271.6.R1614
Wilson-Poe A, Jeong H, Vaughan CW (2017) Chronic morphine reduces the readily releasable pool of GABA, a presynaptic mechanism of opioid tolerance. J Physiol 595(20):6541–6555. https://doi.org/10.1113/JP274157
Xu H, Boychuk JA, Boychuk CR et al (2015) Nicotine enhances inhibition of mouse vagal motor neurons by modulating excitability of premotor GABAergic neurons in the nucleus tractus solitarii. J Neurophysiol 113(4):1165–1174
y Valenzuela IM, Rogers RC, Hermann GE et al (2004) Norepinephrine effects on identified neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol-Gastrointest Liver Physiol 286(2):G333–G339
Zhang X, Fogel R (2002) Glutamate mediates an excitatory influence of the paraventricular hypothalamic nucleus on the dorsal motor nucleus of the vagus. J Neurophysiol 88(1):49–63. https://doi.org/10.1152/jn.2002.88.1.49
Zhang X, Renehan WE, Fogel R (1998) Neurons in the vagal complex of the rat respond to mechanical and chemical stimulation of the GI tract. Am J Physiol-Gastrointest Liver Physiol 274(2):G331–G341. https://doi.org/10.1152/ajpgi.1998.274.2.G331
Zheng ZL, Rogers RC, Travagli RA (1999) Selective gastric projections of nitric oxide synthase-containing vagal brainstem neurons. Neuroscience 90(2):685–694. https://doi.org/10.1016/S0306-4522(98)00586-7
Zhou S, Lu Y, Yao H et al (2008) Spatial organization of neurons in the dorsal motor nucleus of the vagus synapsing with intragastric cholinergic and nitric oxide/VIP neurons in the rat. Am J Physiol-Gastrointest Liver Physiol 294(5):G1201–G1209
Acknowledgements
RG expresses great appreciation to Gail J. Price for her wisdom and backing. This work was supported by the National Institutes of Health (NIDDK, United States) Grant R01-DK117508.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gillis, R.A., Dezfuli, G., Bellusci, L. et al. Brainstem Neuronal Circuitries Controlling Gastric Tonic and Phasic Contractions: A Review. Cell Mol Neurobiol 42, 333–360 (2022). https://doi.org/10.1007/s10571-021-01084-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-021-01084-5