Abstract
The purpose of this study is to examine the hornification of enzymatically hydrolyzed high consistency softwood kraft pulp in an experimental defibration dryer. This device dries pulp under turbulent conditions which can prevent interfiber bonding and produce a separated fiber population. This is useful in certain applications, such as composites, which require dry, unbonded pulp fibers. In this study, we examine how fibrillated pulps behave in the dryer with respect to pore expansion in hydrolysis and collapse in drying (hornification). It was found that the endoglucanase cocktail increased the micro-, meso-, and macropore volumes as a function of hydrolysis time. Drying decreased the pore volumes of each size category, with the biggest changes in the macropore region. The pulp with the highest swelling after hydrolysis had the lowest swelling after drying. The mesopores that were formed in hydrolysis were somewhat preserved after drying. After drying, unfibrillated pulp had good fiber separation, while the highly fibrillated samples formed sub-millimeter, spherical particles.
Similar content being viewed by others
Introduction
Today, many plastic and petrochemical-rich products are attempting to improve their environmental footprint by incorporating more natural materials into their structure (Dadi et al. 2006; Gupta and Verma 2015; Wahlström and Suurnäkki 2015; Wyman et al. 2005). Cellulosic fibers are strong, biodegradable, and with the potential to substitute less sustainable materials. Thus, lignocellulosic pulp fibers have received much attention in both scientific (Arevalo-Gallegos et al. 2017; Barakat et al. 2014; Fatma et al. 2018) and commercial arenas (Alvira et al. 2010; Gupta and Verma 2015; Ingle et al. 2017).
Many applications, such as fiber reinforced polymer composites or air-laid nonwovens (Hegyesi et al. 2019; Santos et al. 2021; Sharma et al. 2019), require dry fibers that are unbonded to each other and in a desirable conformation. These single fibers are usually obtained by the mechanical processing of dried “fluff” pulp sheets. However, the mechanical impact of hammermills or disintegrators can shorten the fiber length, therefore altering the reinforcement provided by fibers within the composites. For this reason, it would be desirable to dry pulp while preventing the fiber bonding and damage.
In a typical pulp drying machine (Vainio and Paulapuro, 2007), interfiber bonds are formed between the fibers as water is removed and consolidation occurs. The fibers enter the pulp dryer in an aqueous suspension, and are formed into dry, bonded sheets for further transport and processing. When water is removed, surface tension pulls the fibers together and eventually forms interfiber hydrogen bonds. Flash drying is an alternative process where pastes, granules and other forms of solids are dried in an air stream, usually with the aid of cyclones and various separation and homogenous stages (Zimmermann et al. 2016). Defibration drying is a type of flash drying where mechanical agitation and turbulent air flow are applied simultaneously to a high consistency pulp suspension. The mechanical stresses overcome surface tension forces and separate individual fibers from the flocs. Thus, moisture is removed and the formation of interfiber bonds is prevented.
The morphology of freely dried fibers can be different from sheet-dried fibers (Ko et al. 2005; Paajanen et al. 2019; Yancey et al. 2003). For example, they can undergo shrinking and twisting. Therefore, it is important to understand how the structure is changed by free drying.
In many water-based pulp applications, such as paper and board production, pulp fibers are treated mechanically to soften and fibrillate the cell wall, increase fibers’ porosity and flexibility, and promote bonding and strength (Liu et al. 2016). The weakening of the internal structure of the fiber cell wall is called internal fibrillation, while the generation of fibrils on the fiber surface is called external fibrillation (Kang and Paulapuro 2006; Wang et al. 2003). Fibrillation increases the strength potential of pulps and is essential for many applications (Liu et al. 2016; Singh et al. 2014; Wang et al. 2007). A number of dry fiber applications, especially composites and nonwovens, could potentially benefit from the increased surface area associated with fibrillation.
While fibrillation is usually achieved mechanically with pulp refiners, it can also be performed chemically. Especially, high consistency enzymatic hydrolysis has received attention in the last few years (Rahikainen et al. 2019, 2020; Zhang et al. 2009) as a method to fibrillate and break down the cell wall in a controlled way. Cellulases, especially endoglucanase (Hammerer et al. 2020), are among the most important β-glucosidases used to depolymerize cellulose. Hydrolysis with cellulase enzymes is a non-toxic and environmentally friendly way to fibrillate pulp fibers, enlarge their effective surface area, and increase their swelling ability (Gehmayr and Sixta 2012; Gourlay et al. 2013; Grethlein 1985; Henriksson et al. 2007; Ibarra et al. 2010; Rahikainen et al. 2019, 2020; Tang et al. 2012; Yang et al. 2019).
Drying fibrillated fibers, while maintaining their high porosity and surface area, is challenging because of the aggregation of microfibrils and collapse of the cell wall pores (Cichosz and Masek 2019; Koo et al. 2020). This process is known as hornification. Hornification is related to the strength loss of pulps and is the main limiting factor in the recycling of low-yield pulps (Duan et al. 2015; Minor 1994; Salmén and Stevanic 2018). The changes to the cell wall pores can be used to quantify both fibrillation and hornification.
In a previous study (Paajanen et al. 2019), it was observed that defibration drying could process unfibrillated kraft pulp into a collection of unbonded, twisted fibers. The present study determines how defibration drying affects the swelling and pore structure of a commercial low-yield pulp which have been enzymatically treated.
Materials and methods
Materials
The pulp used for these experiments was a never dried, bleached softwood (Norway Spruce and Scots Pine) kraft pulp (NDBSK) provided by a Finnish forest industry company. The enzyme used for the treatments was ECOPULP R (AB Enzymes), a cellulase enzyme preparation with main activity endo-1,4-β-D-glucanase produced from Tricoderma reesei.
Enzymatic treatment
NDBSK was wet disintegrated according to SCAN-C 18:65. The pH was adjusted to 5.5-6, and the pulp solids content was increased to 20% w/w by filtration. The high consistency pulp was homogenized in a Kenwood food mixer at room temperature prior to hydrolysis A series of three pulp samples (Series I) was obtained by hydrolyzing pulp for 30, 70, and 180 min at 55 °C with ECOPULP R. The enzyme dosage was 1.5 mg/g pulp (oven-dry basis). The pulps were then placed into plastic bags and kept in an ice bath to deactivate the enzyme. The samples of this first series are referred to as ND-0 (reference), ND-30, ND-70 and ND-180, where ND means never-dried and the number represents the hydrolysis time.
Drying
A second series (Series II) of samples, namely D-0, D-30, D-70 and D-180, was obtained by drying the reference pulp and the enzymatically treated pulps with a home-built lab-scale defibration dryer (Fig. 1). Pulp fibers are mixed by the rotor at the bottom of the drying chamber and agitated and dried by a hot turbulent airflow.
Drying was monitored by measuring the relative humidity (RH, %) of exit air from the top of the drying chamber. The samples were considered dried after 10 s of stable 1% RH, which corresponded to a final pulp solids content of about 90%. An illustration of a drying curve representing drying of never dried samples is shown in Fig. 2. The drying temperature was 45 ℃ and the drying time ranged from 15 to 30 min depending on the sample. For each drying batch, the chamber was loaded with ca. 30 g (oven-dried basis) pulp at ca. 20% solids content. In the case of the most hydrolyzed and stickiest sample, D-180, the lid was opened several times to manually facilitate the homogeneity of the batch.
Schematic representation (A) and a photo of the defibration dryer from outside (B) and from inside (C). The device is composed by two parts: a drying chamber (at the bottom, drawn in black) and a filtration chamber (on top, drawn in blue) separated by a 0.05 mm metal mesh (5). Pulp is mixed by a rotor equipped with two opposing pairs of blades (1) and by a hot turbulent flow. Air is heated with in-line air heaters (AHP Series, Omegalux®) (3), and turbulence is obtained by orienteering the six air inlets. A temperature sensor is connected to each air inlet, and a relative humidity sensor (4) measures the humidity of the air flowing out of the drying chamber. The RH% sensor and the window (2) allow to monitor the drying process. A small fraction of particles can exit the drying chamber, but they are collected by the filtration chamber whose top is made of a 25 μm metal mesh (6)
Drying rate curves of the wet pulps: the reference pulp (ND-0) and the hydrolyzed pulps (ND-30, ND-70, and ND-180). The defibration dryer is connected to an Arduino software that records relative humidity over time. When the relative humidity of the air is stable at 1% for 10 s, the pulp is considered dried
Characterization methods
Degree of polymerization and fiber morphology
The intrinsic viscocity of the samples belonging to Series I was measured according to SCAN-CM 15:99. Furthermore, the degree of polymerization (DP) was calculated using the Mark-Hauwink equation, as suggested in (Ceccherini and Maloney 2019):
where [η] is the intrinsic viscocity and Q′ and α are parameters dependent on the polymer-solvent system. The measurement was performed in duplicates.
The fiber length, width, fibrillation, and fines content were measured by a Kajaani FS-200 Fiberlab Analyser (Metso). Measurements were done in triplicate.
The pulp samples, sputtered with platinum and palladium, were imaged using a Zeiss Sigma VP scanning electron microscope (SEM) equipped with a Schottky FEG emitter. The acceleration voltage of the microscope was set to 2.5 kV. Prior to the analysis, the samples were solvent exchanged and critically point dried (Lovikka et al. 2016; Rahikainen et al. 2020).
Fiber swelling was estimated by the water retention value (WRV). These tests were performed in duplicates according to a modified version of standard SCAN-C 62:00 suitable for highly swollen pulps (Rantanen et al. 2015).
Pore structure and fiber swelling
The pore structure of the samples was characterized by classifying the pore volumes in three categories: micro-, meso- and macropores. In a slight deviation from the IUPAC definition, we separated the three pore categories based on the following pore diameter (D) ranges: micropores: D < 3.2 nm, mesopores: 3.2 < D < 50 nm and macropores: D > 50 nm. The micropores were measured by solute exclusion, the mesopores by N2 gas sorption, and the macropores were calculated subtracting the mesopores from the water retention value.
The solute exclusion technique, ordinally developed by Stone and Scallan (Stone and Scallan 1968a), is based on measuring the water inaccessible to a dextran probe molecule which is assumed to not adsorb or otherwise interact with the fiber surface. In this version of the test (Grönqvist et al. 2014), micropores were measured using a 5000 Dalton dextran with a Stokes diameter of 3.2 nm (T5 by Pharmacosmos).
N2 adsorption was used to measure the mesopore volumes. Given that this technique is applicable only to dried samples, the pulps were critical point dried (CPD) to minimize the shrinkage of the wet pores. Prior to the drying, the samples were solvent-exchanged with dry acetone. Then, acetone was exchanged with liquid CO2 and CO2 was evaporated above its critical point, where its surface tension is negligible. CO2 exchange and drying of the samples were done in Leica EM CPD300. Further details of the exchange protocol can be found in Lovikka et al. (2016). About 50 mg of CPD dried pulps were quickly transferred into the sample tubes of the N2 sorption analyzer (Tristar II by Micromeritics). The adsorption isotherms were used to calculate the mesopore volume from the Barrett, Joyner, and Halenda (BJH) model (Lange et al. 2014). The isotherms were analyzed by the Tristar 3020 software by Micromeritics. The mesopore volume measured by N2 adsorption is hereafter referred to as CPD mesopores.
Results and discussion
Enzymatic fibrillation
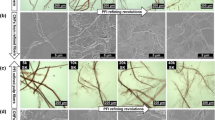
As shown in Table 1, Fig. 3, the enzymatic hydrolysis caused a significant change to pulp DP, fiber morphology and fines content. The DP is lowered, the fibers are cut and fines and external fibrillation increase with longer hydrolysis times. Figure 3 shows that at 0 min hydrolysis time, the fibers are intact and unfibrillated. At 30 min, the surface of the fibers shows extensive external fibrillation. At 70 min the fiber structure has significantly deteriorated. By 180 min, the fiber structure is almost completely destroyed and the sample is converted from a fiber suspension to a high consistency microfibrillated cellulose paste.
The width of cellulases is typically around 4–6 nm (Bubner et al. 2012), while the cell wall pores of low-yield pulp fibers are mostly in the range of 10–30 nm (Stone and Scallan 1967). Thus, Ecopulp R can diffuse into the cell wall. The enzyme attacks the less ordered regions of cellulose and lowers the modulus, allowing the cell wall to swell. In Table 2, the process of “internal fibrillation” increases the expansion of the cell wall across all pore classes. The macropores pass from 1.15 to 3.10, the micropores from 0.35 to 0.85, and the mesopores from 0.33 to 0.46 mL/g. The expansion of the micropores due to the hydrolysis is particularly notable, because usually purely mechanical fibrillation of low yield pulp does not significantly impact on this category of small pores; instead macropores are opened are opened are opened are opened are opened are opened are opened are opened. The increase in micropore volume suggests that the enzyme used here partially loosened up the fibril aggregates (Rahikainen et al. 2020). On the other hand, the increase in macropore volume shows that the enzymatic hydrolysis expanded the space between the cell wall lamellae and partially deaggregated the macrofibrils.
Defibration drying
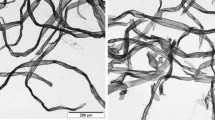
The samples dried in the defibration dryer are shown at a low magnification in Fig. 4 and high magnification in Fig. 5. Without hydrolysis, the fibers are fairly well separated. In Fig. 5(1), curls and kinks introduced by this type of drying are shown. The process of fiber twisting in free drying has been more thoroughly described elsewhere (Gärd and Kemiteknik 2002; Sheng-Hsin and Chan 2005). After 30 min hydrolysis, the fibers are already more difficult to dry separately. The wet, fibrillated fiber surface gives higher interfiber adhesion which the turbulence of drying cannot fully overcome. By 70 min, the individual fiber fragments do not really separate and the aggregates begin to form spherical structures. At 180 min, a collection of mostly sub-millimeter spherical particles is generated. While the separated fibers in Fig. 5(1) have applications in composites, nonwovens and other fiber products, the beads shown in Fig. 5(4) may have uses in cosmetics and other applications of microbeads where plastics are currently used.
In addition to the gross morphological changes the samples undergo in drying, the fibers also hornify. Hornification refers to the loss in swelling of a pulp or cellulosic material when it is dried and rewetted (Diniz et al. 2004; Laivins and Scallan 1993; Stone and Scallan 1968b). The underlying phenomenon in hornification in the aggregation of the elementary fibrils (2–5 nm diameter) into macrofibrils (10–60 nm diameter) which may proceed through an irreversible hydrogen bonding mechanism (Donaldson 2007; Kekäläinen 2016; Klemm et al. 2005). The extent of hornification depends on drying temperature, mechanical processing, chemical environment and other factors (Aghajanzadeh et al. 2022; Giacomozzi and Joutsimo 2015; Kato and Cameron 1999). The loss of swelling greatly affects bonding, rheology and other end-use issues (Ceccherini and Maloney 2019; Forsström 2004). To a certain extent, hornification is the opposite process of internal fibrillation, so it is important to understand how enzymatic fibrillation and hornification in the defibration dryer are related.
The loss in swelling or shrinkage for both the macropores and micropores is shown in Fig. 6. These measurements are performed in the wet state and are somewhat comparable with each other (Khanjani et al. 2017; Lovikka et al. 2016). For the unhydrolyzed sample, there is no reduction in micropore volume, but about 10% shrinkage of macropores. This agrees with earlier studies, which show that hornification of kraft pulps affects predominantly the larger pores (Maloney and Paulapuro 1999; Wang 2006). However, when the samples are hydrolyzed, the pore shrinkage changes. The macropore collapse increases throughout the experiment up to about 90% loss in volume at 180 min. This is largely expected as swollen, large pores have low compression strength and easily collapse from surface tension forces. Micropore collapse, on the other hand, increases from 0 up to a maximum of about 20% at 90 min hydrolysis time, before decreasing.
To gain a better understanding of the changes to the pore structure due to hornification, the samples were analyzed with critical point drying followed by N2 adsorption analysis. CPD attempts to preserve the wet-state pore structure by exchanging the water for CO2, which is then removed above the critical point, where surface tension is near 0. Given that the pore shrinkage caused by CPD is minimum, the pore size distribution (PSD) of the dried samples is assumed to reflect the situation in the wet state, at least relatively. CPD/N2 porosimetry is mostly accurate in the mesopore range (2–50 nm ca.) and gives information on the size of the pore body, as opposed to solute exclusion, which is sensitive to pore opening.
In Fig. 7, the adsorption/desorption isotherms are shown for both the hydrolyzed and dried samples. The PSD calculated from the BJH model are shown in Fig. 8. Type II isotherms with hysteresis, such as these, are typical for pulp fibers (Lovikka et al. 2016). Figures 7A and 8A show the effects of the hydrolysis. Longer hydrolyses lead to higher N2 adsorption at higher relative pressures which correspond to an increase of the macropores. This is a typical pattern of change when pulp fibers are mechanically processed. In this case, pore opening from fibrillation (Fig. 8b) extends into the mesopore range, to 20–30 nm pore diameter. Here the difference between the CPD and the solute exclusion test must be recognized. It is well known in the solute exclusion test, that surface interactions limit the accessibility of probe molecules that are at or near the same size of the pores, skewing the apparent PSD towards smaller pores (Alinee and van de Yen 1997). Therefore, it is possible that expansion of mesopores is detected in the SE-micropore test.
The effect of drying on the samples is shown in Figs. 7B and 8B. The differences in PSD that emerge after hydrolysis are accentuated when the samples are dried. Furthermore, the PSD is shifted to even smaller pores. For example, the pore volume of the ND-0 sample is about 0.24 mL/g, and after 180 min hydrolysis, 0.28 mL/g. After drying, D-0 is 0.13 mL/g compared to 0.32 mL/g for the D-180 sample. So, the differences in mesoporosity are not only preserved, but magnified after hornification in the defibration drier. The pore geometry after both hydrolysis and drying has undergone considerable distortion compared to the unprocessed sample.
Conclusions
The present work investigated the morphology, swelling and PSD of a series of kraft pulps first treated with commercial endoglucanase and then dried with a lab-scale defibration dryer.
The enzymatic hydrolysis caused cellulose chain and fiber cutting, external fibrillation, and cell wall expansion across all pore classes. After 180 min hydrolysis, the fiber structure was nearly completely destroyed and a microfibrillated paste was obtained. Macropore and SE-micropore volumes increased by 170 and 143%, respectively.
Defibration drying of unhydrolyzed samples led to nearly complete fiber separation. However, the drying of more hydrolyzed samples formed nearly spherical sub-millimeter beads. The collapse and shrinkage of the various pore classes in drying/rewetting followed a complex pattern. The collapse of macropores increased as a function of the hydrolysis time, reaching 90% at 180 min treatment time. Micropores formed in the enzymatic fibrillation partially collapse in hornification, but to a lower extent than macropores. The combination of enzymatic hydrolysis conditions and drying control give a route for modulating cellulosic material pore structure across a wide range.
Aavailability of data and material
The authors confirm that the data supporting the findings of this study are available within the article. All of the data and materials are owned by the authors and no permissions are required.
References
Aghajanzadeh S, Fayaz G, Soleimanian Y, Ziaiifar AM, Turgeon SL, Khalloufi S (2022) Hornification: lessons learned from the wood industry for attenuating this phenomenon in plant-based dietary fibers from food wastes. Comp Rev Food Sci Food Saf https://doi.org/10.1111/1541-4337.13047
Alinee B, van de Yen T (1997) Porosity of swollen pulp fibers evaluated by polymer adsorption. In: The fundametals of papermaking materials, transactions of the XIth fundamental research symposium, Cambridge, pp 771–788. https://doi.org/10.15376/frc.1997.2.771
Alvira P, Tomás-Pejó E, Ballesteros M, Technology MNB (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101(13), 4851–4861. https://doi.org/10.1016/j.biortech.2009.11.093
Arevalo-Gallegos A, Ahmad Z, Asgher M, Parra-Saldivar R, Iqbal HMN (2017) Lignocellulose: a sustainable material to produce value-added products with a zero waste approach: a review. Int J Biol Macromol 99:308–318. https://doi.org/10.1016/j.ijbiomac.2017.02.097
Barakat A, Chuetor S, Monlau F, Solhy A, Rouau X (2014) Eco-friendly dry chemo-mechanical pretreatments of lignocellulosic biomass: impact on energy and yield of the enzymatic hydrolysis. Appl Energy 113:97–105. https://doi.org/10.1016/j.apenergy.2013.07.015
Bubner P, Dohr J, Plank H, Mayrhofer C, Nidetzky B (2012) Cellulases dig deep: in situ observation of the mesoscopic structural dynamics of enzymatic cellulose degradation. J Biol Chem 287(4):2759. https://doi.org/10.1074/JBC.M111.257717
Ceccherini S, Maloney T (2019) Assessing wood pulp reactivity through its rheological behavior under dissolution. Cellulose 26(18):9877–9888. https://doi.org/10.1007/S10570-019-02750-0
Cichosz S, Masek A (2019) Cellulose structure and property changes indicated via wetting-drying cycles. Polym Degrad Stabil 167:33–43. https://doi.org/10.1016/j.polymdegradstab.2019.05.033
Dadi A, Varanasi S, Schall CA (2006) Enhancement of cellulose saccharification kinetics using an ionic liquid pretreatment step. Biotechnol Bioeng 95(5), 904–910. https://doi.org/10.1002/bit.21047
de Lange M, Vlugt T, Gascon J, Kapteijn F (2014) Adsorptive characterization of porous solids: error analysis guides the way. Microporous Mesoporous Mater 200:199–215. https://doi.org/10.1016/j.micromeso.2014.08.048
Diniz JF, Gil M, Castro JA (2004) Hornification—its origin and interpretation in wood pulps. Wood Sci Technol 37(6), 489–494. https://doi.org/10.1007/s00226-003-0216-2
Donaldson L (2007) Cellulose microfibril aggregates and their size variation with cell wall type. Wood Sci Technol 41(5):443–460. https://doi.org/10.1007/s00226-006-0121-6
Duan C, Long Y, Li J, Ma X, Ni Y (2015) Changes of cellulose accessibility to cellulase due to fiber hornification and its impact on enzymatic viscosity control of dissolving pulp. Cellulose 22(4):2729–2736. https://doi.org/10.1007/s10570-015-0636-9
Fatma S, Hameed A, Noman M, Ahmed T (2018) Lignocellulosic biomass: a sustainable bioenergy source for the future. Protein Peptide 25:148–163. https://doi.org/10.2174/0929866525666180122144504
Forsström J (2004) Fundamental aspects on the re-use of wood based fibres-porous structure of fibres and ink detachment. Doctoral dissertation, Mid Sweden University, Faculty of Science, Technology and Media, Department of Natural Sciences (FSCN)
Gärd J, Kemiteknik C (2002) The influence of fibre curl on the shrinkage and strength properties of paper MASTER’S THESIS 257. Luleå University of Technology, Luleå
Gehmayr V, Sixta H (2012) Pulp properties and their influence on enzymatic degradability. Biomacromolecules 13(3):645–651. https://doi.org/10.1021/BM201784U
Giacomozzi DE, Joutsimo O (2015) Drying temperature and hornification of industrial never-dried Pinus radiata pulps. 1. Strength, optical, and water holding properties. BioResources 10(3):5791–5808. https://doi.org/10.15376/BIORES.10.3.5791-5808
Gourlay K, Hu J, Arantes V, Andberg M, Saloheimo M, Penttilä M, Saddler J (2013) Swollenin aids in the amorphogenesis step during the enzymatic hydrolysis of pretreated biomass. Bioresour Technol 142:498–503. https://doi.org/10.1016/j.biortech.2013.05.053
Grethlein HE (1985) The effect of pore size distribution on the rate of enzymatic hydrolysis of cellulosic substrates. Bio/Technology 3(2):155–160. https://doi.org/10.1038/nbt0285-155
Grönqvist S, Hakala T, Kamppuri T, Cellulose MV (2014). Fibre porosity development of dissolving pulp during mechanical and enzymatic processing. Cellulose 21(5), 3667–3676. https://doi.org/10.1007/s10570-014-0352-x
Gupta A, Verma JP (2015) Sustainable bio-ethanol production from agro-residues: a review. Renew Sustain Energy Rev 41:550–567. https://doi.org/10.1016/j.rser.2014.08.032
Hammerer F, Ostadjoo S, Friščić T, Auclair K (2020) Towards Controlling the reactivity of enzymes in Mechanochemistry: Inert Surfaces protect β-Glucosidase activity during ball milling. Chemsuschem 13(1):106–110. https://doi.org/10.1002/CSSC.201902752
Hegyesi N, Zhang Y, Kohári A, Polyák P, Sui X, Pukanszky B (2019) Enzymatic degradation of PLA/cellulose nanocrystal composites. Ind Crops Prod 141:111799. https://doi.org/10.1016/j.indcrop.2019.111799
Henriksson M, Henriksson G, Berglund LA, Lindström T (2007) An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur Polym J 43(8):3434–3441. https://doi.org/10.1016/j.eurpolymj.2007.05.038
Ibarra D, Köpcke V, Ek M (2010) Behavior of different monocomponent endoglucanases on the accessibility and reactivity of dissolving-grade pulps for viscose process. Enzym Microb Technol 47(7):355–362. https://doi.org/10.1016/j.enzmictec.2010.07.016
Ingle AP, Rathod J, Pandit R, da Silva SS, Rai M (2017) Comparative evaluation of free and immobilized cellulase for enzymatic hydrolysis of lignocellulosic biomass for sustainable bioethanol production. Cellulose 24(12):5529–5540. https://doi.org/10.1007/S10570-017-1517-1
Kang T, Paulapuro H (2006) New mechanical treatment for chemical pulp. Proc Inst Mech Eng Part E J Process Mech Eng 220(3), 161–166. doi: 10.1243/09544089JPME81.
Kato KL, Cameron RE (1999) A review of the relationship between thermally-accelerated ageing of paper and hornification. Cellulose 6(1):23–40. https://doi.org/10.1023/A:1009292120151
Kekäläinen K (2016) Microfibrillation of pulp fibres: the effects of compression-shearing, oxidation and thermal drying. Master’s thesis C586, University of Oulu
Khanjani P, Väisänen S, Lovikka V, Nieminen K, Maloney T, Vuorinen T (2017) Assessing the reactivity of cellulose by oxidation with 4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxo-piperidinium cation under mild conditions. Carbohydr Polym 176:293–298. https://doi.org/10.1016/J.CARBPOL.2017.08.092
Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44(22):3358–3393. https://doi.org/10.1002/ANIE.200460587
Ko Y, Hu S, Makoui KB (2005) Wood pulp fiber morphology modifications through thermal drying. U.S. Patent No. 6,837,970. Google Patents
Koo B, Jo J, Cho SM (2020) Drying effect on enzymatic hydrolysis of cellulose associated with porosity and crystallinity. Appl Sci. https://doi.org/10.3390/app10165545
Laivins G, Scallan A (1993) The mechanism of hornification of wood pulps. Prod Papermak 2:1235
Liu W, Wang B, Hou Q, Chen W, Wu M (2016) Effects of fibrillation on the wood fibers’ enzymatic hydrolysis enhanced by mechanical refining. Bioresour Technol 206:99–103. https://doi.org/10.1016/j.biortech.2016.01.074
Lovikka VA, Khanjani P, Väisänen S, Vuorinen T, Maloney TC (2016) Porosity of wood pulp fibers in the wet and highly open dry state. Microporous Mesoporous Mater 234:326–335. https://doi.org/10.1016/j.micromeso.2016.07.032
Maloney T, Paulapuro H (1999) The formation of pores in the cell wall. J Pulp Pap Sci 25:430–436
Minor J (1994) Hornification-its origin and meaning. Prog Pap Recycl 3(2):93–95
Paajanen A, Ceccherini S, Maloney T, Ketoja JA (2019) Chirality and bound water in the hierarchical cellulose structure. Cellulose 26(10):5877–5892. https://doi.org/10.1007/S10570-019-02525-7
Rahikainen J, Ceccherini S, Molinier M, Holopainen-Mantila U, Reza M, Väisänen S, Puranen T, Kruus K, Vuorinen T, Maloney T, Suurnäkki A, Grönqvist S (2019) Effect of cellulase family and structure on modification of wood fibres at high consistency. Cellulose 26(8):5085–5103. https://doi.org/10.1007/S10570-019-02424-X
Rahikainen J, Mattila O, Maloney T, Lovikka V, Kruus K, Suurnäkki A, Grönqvist S (2020) High consistency mechano-enzymatic pretreatment for kraft fibres: effect of treatment consistency on fibre properties. Cellulose 27(9):5311–5322. https://doi.org/10.1007/S10570-020-03123-8
Rantanen J, Dimic-Misic K, Kuusisto J, Maloney T (2015) The effect of micro and nanofibrillated cellulose water uptake on high filler content composite paper properties and furnish dewatering. Researchgate Net 22(6):4003–4015. https://doi.org/10.1007/s10570-015-0777-x
Salmén L, Stevanic JS (2018) Effect of drying conditions on cellulose microfibril aggregation and “hornification. Cellulose 25(11):6333–6344. https://doi.org/10.1007/S10570-018-2039-1
Santos, A. S., Ferreira, P. J. T., Maloney, T. (2021). Bio-based materials for nonwovens. Cellulose, 28(14), 8939-8969. https://doi.org/10.1007/s10570-021-04125-w
Sharma A, Thakur M, Bhattacharya M, Mandal T, Goswami S (2019) Commercial application of cellulose nano-composites – a review. Biotechnol Rep (Vol 21:e00316. https://doi.org/10.1016/j.btre.2019.e00316
Hu S-H, Chan KY (2005) Method of producing twisted, curly fibers (United states/ US-7364639-B2). KIMBERLY CLARK CO (US). https://patents.google.com/patent/US7364639B2
Singh R, Bhardwaj NK, Choudhury B (2014) An experimental study of the effect of enzyme-assisted refining on energy consumption and paper properties for mixed hardwood pulp. Appita J (Vol 67(3):226–231
Stone JE, Scallan AM (1967) The effect of component removal upon the porous structure of the Cell Wall of Wood. II. Swelling in Water and the Fiber saturation point. Tappi 50:496–501. https://doi.org/10.32964/TJ50.10.496
Stone JE, Scallan AM (1968) A structural model for the cell wall of water-swollen wood pulp fibres based on their accessibility to macromolecules. Cell Chem Technol 2:343–358
Tang Y, Huang GW, Xia J, Zue GX, Zhang Y (2012). Effect of cellulase enzyme treatment on the pulp beatability and fiber properties. Trans Tech Publ, 441, 746–749
Vainio AK, Paulapuro H, Interfiber bonding and fiber segment activation in paper. BioResources (2007) 2(3), 442–458
Wahlström RM, Suurnäkki A (2015) Enzymatic hydrolysis of lignocellulosic polysaccharides in the presence of ionic liquids. Green Chem 17(2):694–714. https://doi.org/10.1039/C4GC01649A
Wang X (2006) Improving the papermaking properties of kraft pulp by controlling hornification and internal fibrillation. http://aaltodoc.aalto.fi/handle/123456789/2749
Wang X, Maloney TC, Paulapuro H (2003) Internal fibrillation in never-dried and once-dried chemical pulps. Appita J 56(6):455–459
Wang X, Maloney TC, Paulapuro H (2007) Fibre fibrillation and its impact on sheet properties. Paperi Ja Puu Pap Timber 89:148–151
Wyman CE, Dale BE, Elander RT, Holtzapple M, Ladisch MR, Lee YY (2005) Coordinated development of leading biomass pretreatment technologies. Bioresour Technol 96:1959–1966 https://doi.org/10.1016/j.biortech.2005.01.010
Yancey M, Wester B, Vrbanac M, Dezutter R (2003) Dried singulated crosslinked cellulose pulp fibers. Google Patents
Yang S, Yang B, Duan C, Fuller DA, Wang X, Chowdhury SP, Stavik J, Zhang H, Ni Y (2019) Applications of enzymatic technologies to the production of high-quality dissolving pulp: a review. Bioresour Technol 281:440–448. https://doi.org/10.1016/J.BIORTECH.2019.02.132
Zhang X, Qin W, Paice MG, Saddler JN (2009) High consistency enzymatic hydrolysis of hardwood substrates. Bioresour Technol 100(23):5890–5897. https://doi.org/10.1016/j.biortech.2009.06.082
Zimmermann MVG, Borsoi C, Lavoratti A, Zanini M, Zattera AJ, Santana RMC (2016) Drying techniques applied to cellulose nanofibers. J Reinf Plast Compos 35(8):682–697. https://doi.org/10.1177/0731684415626286
Acknowledgments
Not applicable.
Funding
Open Access funding provided by Aalto University. The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
DD performed the experimental part. All the authors contributed equally to the writing part of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Consent for publication
All the authors gave their consent to publish this work.
Consent to participate
Not Applicable.
Ethics approval
Not Applicable.
Human and animal rights statement
This research did not involve any Human Participants and/or Animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dahiya, D., Ceccherini, S. & Maloney, T.C. Impact of high consistency enzymatic hydrolysis and defibration drying on cellulose fiber pore characteristics. Cellulose 30, 7607–7618 (2023). https://doi.org/10.1007/s10570-023-05398-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05398-z