Abstract
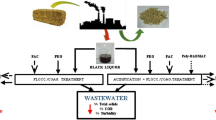
In the lyocell process, N-methylmorpholine-N-oxide (NMMO) with a high recovery rate is used as a solvent to directly dissolve cellulose, which is of great significance for the realization of industrialization. In this paper, the impurity removal process and side reactions in the NMMO recovery system are introduced. The NMMO recovery system is generally composed of three major processes: air flotation, ion exchange and evaporation. Air flotation and ion exchange are the processes of removing impurities. And evaporation, where side reactions occur, is the process of increasing the concentration of NMMO. In terms of conductivity and colority, organic flocculants are better than inorganic flocculants in the air flotation process which mainly removes undissolved particulate matter, part of hemicelluloses and some colored substances. The ion exchange process mainly removes ionic substances and propyl gallate (PG). Side reactions occur during evaporation, producing colored substances and black particulate matter. Through analysis, it can be known that the production of colored substances is caused by the condensation reaction of N-methylmorpholine (NMM, 1), morpholine (M, 2), 2-Amino-4-methyl-oxazole (3), furfuryl alcohol (4), Morpholinoacetonitrile (5), xylose and NMMO under alkaline and high-temperature conditions, rather than by PG. The black particles are due to thermal degradation of NMMO or NMM or M in the presence of oxygen and iron at high temperatures.




Similar content being viewed by others
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
References
Adorjan I, Potthast A, Rosenau T, Sixta H, Kosma P (2005) Discoloration of cellulose solutions in N-methylmorpholine-N-oxide (lyocell). Part 1: studies on model compounds and pulps. Cellulose 12(1):51–57
Bernier D, Wefelscheid UK, Woodward S (2009) Properties, preparation and synthetic uses of amine N-oxides. An Update Org Prep Proc Int 41(3):175–210
Ferris JP, Gerwe RD, Gapski GR (1967) Detoxication mechanisms. II. Iron-catalyzed dealkylation of trimethylamine oxide. J Am Chem Soc 89(20):5270–5275
Ferris JP, Gerwe RD, Gapski GR (1968) Detoxication mechanisms. III. The scope and mechanism of the iron-catalyzed dealkylation of tertiary amine oxides. J Org Chem 33(9):3493–3498
Ghafari S, Aziz HA, Isa MH, Zinatizadeh AA (2009) Application of response surface methodology (RSM) to optimize coagulation–flocculation treatment of leachate using poly-aluminum chloride (PAC) and alum. J Hazard Mater 163(2–3):650–656
Konkin A, Wendler F, Meister F, Roth H, Aganov A, Ambacher O (2007) Degradation processes in the cellulose/N-methylmorpholine-N-oxide system studied by HPLC and ESR. Radical formation/recombination kinetics under UV photolysis at 77 K. Cellulose 14(5):457–468
Nelan DR, Robeson CD (1962) The oxidation product from α-tocopherol and potassium ferricyanide and its reaction with ascorbic and hydrochloric acids. J Am Chem Soc 84(15):2963–2965
Pieterse T (2013) N-Methylmorpholine-N-Oxide Synlett 24(16):2175–2176
Potthast A, Rosenau T, Kosma P (2006) Analysis of oxidized functionalities in cellulose. Adv Polym Sci 205:1–48
Rosenau T, Potthast A, Kosma P, Chen CL, Gratzl JS (1999) Autocatalytic decomposition of N-methylmorpholine N-oxide induced by Mannich intermediates. J Org Chem 64(7):2166–2167
Rosenau T, Potthast A, Sixta H, Kosma P (2001) The chemistry of side reactions and byproduct formation in the system NMMO/cellulose (lyocell process). Progr Polym Sci 26(9):1763–1837
Rosenau T, Potthast A, Adorjan I, Hofinger A, Sixta H, Firgo H, Kosma P (2002a) Cellulose solutions in N-methylmorpholine-N-oxide (NMMO)–degradation processes and stabilizers. Cellulose 9(3–4):283–291
Rosenau T, Potthast A, Hofinger A, Sixta H, Kosma P (2002b) Instabilities in the system NMMO/water/cellulose (lyocell process) caused by Polonowski type reactions. Holzforschung 56(2):199–208
Rosenau T, Hofinger A, Potthast A, Kosma P (2003) On the conformation of the cellulose solvent N-methylmorpholine-N-oxide (NMMO) in solution. POLYMER-LONDON- 44(20):6153–6158
Rosenau T, Potthast A, Milacher W, Adorjan I, Hofinger A, Kosma P (2005) Discoloration of cellulose solutions in N-methylmorpholine-N-oxide (lyocell). Part 2: isolation and identification of chromophores. Cellulose 12(2):197–208
Shiba R, Takahashi M, Ebisuno T, Takimoto M (1993) Reaction of N″-cyanoguanidine with formaldehyde (VII). Preparation of new flocculants for anionic colloidal particles. Bull Chem Soc Jpn 66(8):2452–2453
Sohn OS, Fiala ES, Conaway CC, Weisburger JH (1982) Separation of morpholine and some of its metabolites by high-performance liquid chromatography. J Chromatogr A 242(2):374–380
Xie W, Gong Y, Yu K (2017) Determination of total sugar content in lignocellulosic hydrolysates by using a reaction headspace gas chromatographic technique. Cellulose 24(11):4591–4597
Zeng T, Ke X, Li L, Cheng X, Ni Y, Ouyang X, Zhang X, Chen L, Huang L, Hu H (2020) Quantification of N-methylmorpholine N-oxide in biorefinery process solution by headspace gas chromatography. Cellulose 27:6861–6870
Zhang S, Ke X, Zeng T, Ni Y, Luo X, Hu H, Chen L, Huang L (2020) Determination of N-Methyl morpholine in biomass pretreatment solutions by the ammonia-assisted headspace gas chromatography. Renew Energy 145:2380–2386
Acknowledgments
The authors gratefully acknowledge financial support from the National Key Research and Development Program of China (Grant No. 2017YFB0309502).
Funding
This work was supported by the National Key Research and Development Program of China (No. 2017YFB0309502).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by YG, JC, TS, LX, CC, KC, JX and TL. The first draft of the manuscript was written by YG and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, Y., Cai, J., Sun, T. et al. The purification process and side reactions in the N-methylmorpholine-N-oxide (NMMO) recovery system. Cellulose 28, 7609–7617 (2021). https://doi.org/10.1007/s10570-021-03929-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-021-03929-0