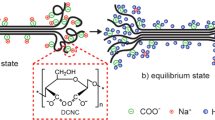
Abstract
A novel cellulose-based polyampholyte derivative, carboxylethyl quaternized cellulose (CEQC), was homogeneously synthesized by introducing positively charged quaternary ammonium groups and negatively charged carboxyl groups to the backbone of cellulose. The structure and dilute solution properties of CEQCs were characterized with elemental analysis, FTIR, NMR, viscometer, light scattering and zeta-potential measurement. The nitrogen content and total degree of substituent of acylamino and carboxyl groups increased with an increase of the molar ratio of acrylamide to the anhydroglucose unit of quaternized cellulose (QC). The salt-resistance of CEQC was improved remarkably by introducing opposite charged carboxyl to the QC chains. The intrinsic viscosity of the prepared polyampholytes was found to be very sensitive to the pH of the solutions. CEQC-1, the sample with relative low content of carboxyl groups, behaved as a classical cationic polyelectrolyte. However, CEQC-2 and CEQC-3, the samples with higher content of carboxyl groups, displayed typical polyampholyte behavior, and the isoelectric points (IEP) were determined to be 5.0 and 3.8 respectively. This work provided a facile method for the synthesis of novel cellulose-based polyampholytes with different IEP.








Similar content being viewed by others
References
Alfrey T, Morawetz H, Fitzgerald EB, Fuoss RM (1950) Synthetic electrical analog of proteins. J Am Chem Soc 72:1864
Annenkov VV, Danilovtseva EN, Saraev VV, Mikhaleva AI (2003) Complexation of copper(II) ions with imidazole-carboxylic polymeric systems. J Polym Sci Part A Polym Chem 41:2256–2263
Bekturov EA, Kudaibergenov SE, Rafikov SR (1990) Synthetic polymeric ampholytes in solution. J Macromol Sci Polym Rev 30:233–303
Brown W, Wiskstön R (1965) A viscosity-molecular weight relationship for cellulose in cadoxen and a hydrodynamic interpretation. Eur Polym J 1:1–10
Candau F, Joanny JF (1996) Polymeric materials encyclopedia. CRC, Boca Raton, FL, pp 5476–5488
Chen WI, Alexandridis P, Su CK, Patrickios CS, Hertler WR, Hatton TA (1995) Controlled living radical polymerization halogen atom transfer radical polymerization promoted by a Cu(I)Cu(II) redox process. Macromolecules 28:8604–8611
Chen L, Tian Z, Du Y (2004) Synthesis and pH sensitivity of carboxymethyl chitosan-based polyampholyte hydrogels for protein carrier matrices. Biomaterials 25:3725–3732
Chen Y, Liu Y, Tang H, Tan H (2010) Study of carboxymethyl chitosan based polyampholyte superabsorbent polymer I: optimization of synthesis conditions and pH sensitive property study of carboxymethyl chitosan-g-poly(acrylic acid-co-dimethyldiallylammonium chloride) superabsorbent polymer. Carbohydr Polym 81:365–371
Edwards SF, King PR, Pincus P (1980) Phase changes in polyampholytes. Ferroelectrics 30:3–6
Ezell RG, McCormick CL (2007) Electrolyte- and pH-responsive polyampholytes with potential as viscosity-control agents in enhanced petroleum recovery. J Appl Polym Sci 104:2812–2821
Haack V, Heinze T, Oelmeyer G, Kulicke WM (2002) Starch derivatives of high degree of functionalization, 8. Synthesis and flocculation behavior of cationic starch polyelectrolytes. Macromol Mater Eng 287:495–502
Halverson F, Lancaster JE, O’Conner MN (1985) Sequence distribution of carboxyl groups in hydrolyzed polyacrylamide. Macromolecules 18:1139–1144
Hampton KW Jr, Ford WT (2000) Styrylmethyl(trimethyl)ammonium methacrylate polyampholyte latexes. Macromolecules 33:7292–7299
Heinze T, Genco T (2012) Synthesis and characterization of aminocellulose sulfates as novel ampholytic polymers. Cellulose 19:1305–1313
Huang JT, Zhang J, Zhang JQ, Zheng SH (2005) Template imprinting amphoteric polymer for the recognition of proteins. J Appl Polym Sci 95:358–361
Ji E, Whitten DG, Schanze KS (2011) pH-dependent optical properties of a poly(phenylene ethynylen) conjugated polyampholyte. Langmuir 27:1565–1568
Kamachi M, Kurihara M, Stille JK (1972) Synthesis of block polymers for desalination membranes. Preparation of block copolymers of 2-vinylpyridine and methacrylic acid or acrylic acid. Macromolecules 5:161–167
Khokhlov AR, Nyrkova IA (1992) Compatibility enhancement and microdomain structuring in weakly charged polyelectrolyte mixtures. Macromolecules 25:1493–1502
Klemm D, Heubletin B, Fink HP, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44:3358–3393
Korchagina EV, Philippova OE (2010) Multichain aggregates in dilute solutions of associating polyelectrolyte keeping a constant size at the increase in the chain length of individual macromolecules. Biomacromolecules 11:3457–3466
Kudaibergenov SE, Ciferri A (2007) Natural and synthetic polyampholytes, 2. Functions and applications. Macromol Rapid Commun 28:1969–1986
Li S, Wu Y, Wang J, Zhang Q, Kou Y, Zhang S (2010) Double-responsive polyampholyte as a nanoparticle stabilizer: application to reversible dispersion of gold nanoparticles. J Mater Chem 20:4379–4384
Loubaki E, Ourevitch M, Sicsic S (1991) Chemical modification of chitosan by glycidyl trimethylammonium chloride. Characterization of modified chitosan by 13C- and 1H-NMR spectroscopy. Eur Polym J 27:311–317
Lowe AB, McCormick CL (2002) Synthesis and solution properties of zwitterionic polymers. Chem Rev 102:4177–4189
Mahltig B, Cheval N, Gohy J, Fahmi A (2010) Preparation of gold nanoparticles under presence of the diblock polyampholyte PMAA-b-PDMAEMA. J Polym Res 17:579–588
Mao M, Turner SR (2007) Aggregation of rod-coil block copolymers containing rigid polyampholyte blocks in aqueous solution. J Am Chem Soc 129:3832–3833
Mayadunne RTA, Rizzardo E, Chiefari J, Krstina J, Moad G, Postma A, Thang SH (2000) Living polymers by the use of trithiocarbonates as reversible addition-fragmentation chain transfer (RAFT) agents: ABA triblock copolymers by radical polymerization in two steps. Macromolecules 33:243–245
McCormick CL, Kathmann EE (1996) Polymeric materials encyclopedia. CRC, Boca Raton, FL, pp 5462–5476
McCormick CL, Salazar LC (1992) Water-soluble copolymers. 43. Ampholytic copolymers of sodium 2-(acrylamido)-2-methylpropanesulfonate with [2-(acrylamido)-2-methylpropyl]trimethylammonium chloride. Macromolecules 25:1896–1900
Nisato G, Munch JP, Candau SJ (1999) Swelling, structure, and elasticity of polyampholyte hydrogels. Langmuir 15:4236–4244
Noh JG, Sung YJ, Geckeler KE, Kudaibergenov SE (2005) Synthesis, characterization, and stimuli-sensitive properties of novel polycarbobetaines. Polymer 46:2183–2190
Osada Y, Gong JP (1998) Soft and wet materials polymer gels. Adv Mater 10:827–837
Pafiti KS, Philippou Z, Loizou E, Porcar L, Patrickios CS (2011) End-linked poly[2-(dimethylamino)ethyl methacrylate]–poly(methacrylic acid) polyampholyte conetworks: synthesis by sequential RAFT polymerization and swelling and SANS characterization. Macromolecules 44:5352–5362
Patrickios CS, Yamasaki EN (1995) Polypeptide amino acid composition and isoelectric point II. Comparison between experiment and theory. Anal Chem 231:82–91
Patrickios CS, Hertler WR, Abbott NL, Hatton TA (1994) Diblock, ABC triblock, and random methacrylic polyampholytes: synthesis by group transfer polymerization and solution behavior. Macromolecules 27:930–937
Patrickios CS, Sharma LR, Armes SP, Billingham NC (1999) Precipitation of a water soluble ABC triblock methacrylic polyampholyte effects of time, pH, polymer concentration, salt type and concentration, and presence of a protein. Langmuir 15:1613–1620
Ribeiro JM, Sillero A (1991) A program to calculate the isoelectric point of macromolecules. Comput Biol Med 21:131–141
Sillero A, Ribeiro JM (1989) Isoelectric points of proteins: theoretical determination. Anal Biochem 179:319–325
Šimkovic I, Synytsya A, Uhliariková I, Copíková J (2009) Amidated pectin derivatives with n-propyl-, 3-aminopropyl-, 3-propanol- or 7-aminoheptyl- substituents. Carbohydr Polym 76:602–606
Song Y, Sun Y, Zhang X, Zhou J, Zhang L (2008a) Homogeneous quaternization of cellulose in NaOH/urea aqueous solutions as gene carriers. Biomacromolecules 9:2259–2264
Song Y, Zhou J, Zhang L, Wu X (2008b) Homogenous modification of cellulose with acrylamide in NaOH/urea aqueous solutions. Carbohydr Polym 73:18–25
Tan B, Ravi P, Tam KC (2006) Synthesis and characterization of novel pH-responsive polyampholyte microgels. Macromol Rapid Commun 27:522–528
Tanford Ch (1961) Physical chemistry of macromolecules. Wiley, New York
Tsitsilianis C, Stavrouli N, Bocharova V, Angelopoulos S, Kiriy A, Katsampas I, Stammc M (2008) Stimuli responsive associative polyampholytes based on ABCBA pentablock terpolymer architecture. Polymer 49:2996–3006
Vasconcelos CL, Bezerril PM, Santos DES, Dantas TNC, Pereira MR, Fonseca JLC (2006) Effect of molecular weight and ionic strength on the formation of polyelectrolyte complexes based on poly(methacrylic acid) and chitosan. Biomacromolecules 7:1245–1252
Vasilevskaya VV, Potemkin II, Khokhlov AR (1999) Swelling and collapse of physical gels formed by associating telechelic polyelectrolytes. Langmuir 15:7918–7924
Wang JS, Matyjaszewski K (1995) Control1ed “living” radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes. J Am Chem Soc 117:5614–5615
Yang F, Li G, He Y, Ren F, Wang G (2009) Synthesis, characterization, and applied properties of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohydr Polym 78:95–99
Yin Q, Li Y, Yin Q, Miao X, Jiang B (2009) Synthesis and rheological behavior of a novel N-sulfonate ampholyte chitosan. J Appl Polym Sci 113:3382–3387
Yu H, Huang Y, Ying H, Xiao C (2007) Preparation and characterization of a quaternary ammonium derivative of konjac glucomannan. Carbohydr Polym 69:29–40
Zhang Y, Li S, Zhang L (2010) Aggregation behavior of triple helical polysaccharide with low molecular weight in diluted aqueous solution. J Phys Chem B 114:4945–4954
Zurimendi JA, Guerrero SJ, Leon V (1984) The determination of the degree of hydrolysis in poly(acrylamides): simple methods using C13 n.m.r., and elementary analysis. Polymer 25:1314–1316
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (50973085), Program for New Century Excellent Talents in University (NCET-11-0415), National Basic Research Program of China (973 Program, 2010CB732203) and Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
You, J., Hu, H. & Zhou, J. Synthesis, structure and solution properties of the novel polyampholytes based on cellulose. Cellulose 20, 1175–1185 (2013). https://doi.org/10.1007/s10570-013-9891-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-013-9891-9