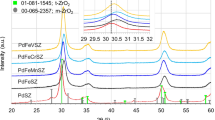
We studied on the function of the metal in the sulfated zirconia(SO 2−4 /ZrO2) catalyst for the isomerization reaction of light paraffins. The addition of Pt to the SO 2−4 /ZrO2 carrier could keep the high catalytic activity. The improvement in this isomerization activity is because Pt promotes removal of the coke precursor deposited on the catalyst surface. Though this catalytic function was observed in other transition metals, such as Pd, Ru, Ni, Rh and W, Pt exhibited the highest effect among them. It was further found that the Pd/SO 2−4 /ZrO2–Al2O3 catalyst possessed a catalytic function for desulfurization of sulfur-containing light naphtha in addition to the skeletal isomerization. The sulfur tolerance of catalyst depended on the method of adding Pd, and the catalyst prepared by impregnation of the SO 2−4 /ZrO2–Al2O3 with an aqueous solution of Pd exhibited the highest sulfur tolerance.
Further, we investigated the improvement in sulfur tolerance of the Pt/SO 2−4 /ZrO2–Al2O3 catalyst by impregnation of Pd. The results of EPMA analysis indicated that this catalyst was a hybrid-type one (Pt/SO 2−4 /ZrO2–Pd/Al2O3) in which Pt/SO 2−4 /ZrO2 particles and Pd/Al2O3 particles adjoined closely. This hybrid catalyst possessed a very high sulfur tolerance to the raw light naphtha that was obtained from the atmospheric distillation apparatus, although this light naphtha contained much sulfur. We assume that such a high sulfur tolerance in the hybrid catalyst is brought about by the isomerization function of Pt/SO 2−4 /ZrO2 particles and the hydrodesulfurization function of Pd/Al2O3 particles. Besides, since the hybrid catalyst also provides high catalytic activity in the isomerization of HDS light naphtha, we suggest that the Pd/Al2O3 particles supply atomic hydrogen to the Pt/SO 2−4 /ZrO2 particles by homolytic dissociation of gaseous hydrogen and also enhance the sulfur tolerance of Pt/SO 2−4 /ZrO2 particles. Finally, we also propose the most suitable location of Pd and Pt in the metal-supported SO 2−4 /ZrO2–Al2O3 catalyst.
Similar content being viewed by others
References
N.A. Cusher, A.S. Xarchy, T.C. Sager and M.E. Reno, NPRA Annual Meeting AM-90–35 (1990)
P.J. Kucher J.C. Bricker M.E. Reno R.S. Haizmann (1993) Fuel Process. Technol. 35 IssueID1–2 183 Occurrence Handle10.1016/0378-3820(93)90091-H
A. Hollò J. Hancsók J. Gergeely J. Perger (2000) Conference Proceedings Hungarian Journal of Industrial Chemistry Veszprém 2 16–20
Weyda H. Koehler E. (2003) Catal. Today 81 51 Occurrence Handle10.1016/S0920-5861(03)00101-9
M.J. Cleveland and C.D. Gosling, NPRA Annual Meeting AM-99–29 (1999)
H.W. Kouwenhoven W.C. Langhout (1971) Chem Eng Progress 67 IssueID4 65 Occurrence Handle1:CAS:528:DyaE3MXhsVCjtrk%3D
P.J. Kucher J.C. Bricker M.E. Reno R.S. Haizmann (1993) Fuel Process. Technol 35 IssueID1–2 183 Occurrence Handle10.1016/0378-3820(93)90091-H
T. Kimura (2003) Catal. Today 81 57 Occurrence Handle10.1016/S0920-5861(03)00102-0 Occurrence Handle1:CAS:528:DC%2BD3sXktlyrtr4%3D
T. Hosoi T. Shimizu S. Itoh S. Baba H. Takaoka T. Imai N. Yokoyama (1988) ACS Div. Petrol. Chem. 33 562 Occurrence Handle1:CAS:528:DyaL1MXis1emtw%3D%3D
C. Gosling, R. Rossin, P. Bullen, T. Shimizu and T. Imai, Petrol. Technol. Quart. 55 (1997/1998)
T. Kimura (2002) PETROTECH 25 IssueID2 111 Occurrence Handle1:CAS:528:DC%2BD38Xhsleku7g%3D
R.M. Jao T.B. Lin J.R. Chang (1996) J. Catal. 161 222 Occurrence Handle10.1006/jcat.1996.0180 Occurrence Handle1:CAS:528:DyaK28XksFGjurk%3D
M.A. Arribas F. Marquez A. Martinez (2000) J. Catal. 190 IssueID2 309 Occurrence Handle10.1006/jcat.2000.2768 Occurrence Handle1:CAS:528:DC%2BD3cXhsFemsrs%3D
J.M. Serra A. Chica A. Corma (2003) Appl. Catal. A: Gen 239 35 Occurrence Handle10.1016/S0926-860X(02)00371-X Occurrence Handle1:CAS:528:DC%2BD3sXms1egsw%3D%3D
K. Watanabe T. Kawakami K. Baba M. Oshio T. Kimura (2004) J. Jpn. Petrol. Inst. 47 IssueID2 143 Occurrence Handle10.1627/jpi.47.143 Occurrence Handle1:CAS:528:DC%2BD2cXhvFOrs7Y%3D
T. Okuhara (2004) J. Jpn. Petrol. Inst. 47 IssueID1 1 Occurrence Handle10.1627/jpi.47.1 Occurrence Handle1:CAS:528:DC%2BD2cXpvV2r
H. Matsuhashi H. Shibata H. Nakamura K. Arata (1999) Appl. Catal. A: Gen. 187 99 Occurrence Handle10.1016/S0926-860X(99)00194-5 Occurrence Handle1:CAS:528:DyaK1MXmt1Wrtrc%3D
K. Ebitani J. Konishi H. Hattori (1991) J. Catal. 130 257 Occurrence Handle10.1016/0021-9517(91)90108-G Occurrence Handle1:CAS:528:DyaK3MXktFWht7g%3D
C. Morterra G. Gerrato F. Pinna M. Signoretto G. Strukul (1994) J. Catal. 149 181 Occurrence Handle10.1006/jcat.1994.1283 Occurrence Handle1:CAS:528:DyaK2cXmtlCitLw%3D
H. Liu G.D. Lei W.M.H. Sachtler (1996) Appl. Catal. A: Gen. 146 165 Occurrence Handle10.1016/0926-860X(96)00031-2 Occurrence Handle1:CAS:528:DyaK28Xmt1ags78%3D
K. Watanabe T. Kawakami K. Baba T. Kimura (2004) J. Jpn. Petrol. Inst. 47 IssueID5 348 Occurrence Handle10.1627/jpi.47.348 Occurrence Handle1:CAS:528:DC%2BD2cXnsVKnu78%3D
C. Song (1998) Prep. Am. Chem. Soc. Div. Petrol. Chem. 43 IssueID2 301 Occurrence Handle1:CAS:528:DyaK1cXitlSnu7o%3D
P. Raybaud A. Patrigeon H. Toulhoat (2001) J. Catal. 197 98 Occurrence Handle10.1006/jcat.2000.3064 Occurrence Handle1:CAS:528:DC%2BD3cXptVGntb8%3D
K. Watanabe T. Kawakami K. Baba T. Kimura (2004) Appl. Catal. A: Gen 276 145 Occurrence Handle10.1016/j.apcata.2004.07.055 Occurrence Handle1:CAS:528:DC%2BD2cXovVyqtLY%3D
F. Fujimoto K. Maeba K. Aimoto (1992) Appl. Catal. A: Gen. 91 81 Occurrence Handle10.1016/0926-860X(92)85067-L Occurrence Handle1:CAS:528:DyaK3sXisVSkug%3D%3D
K. Fujimoto (1995) Stud. Surf. Sci. Catal 127 37
W. Qian Y. Yoda Y. Hirai A. Ishihara T. Kabe (1999) Appl. Catal A: Gen. 184 IssueID1 81 Occurrence Handle10.1016/S0926-860X(99)00083-6 Occurrence Handle1:CAS:528:DyaK1MXjsFKntb0%3D
K. Watanabe, K. Baba, T. Kawakami and T. Kimura, The 4th Middle East Refining & Petrochemicals Conference and Exhibition TEC112 (2003)
A. Ishihara (1994) CCT J 45 IssueID9 8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watanabe, K., Kawakami, T., Baba, K. et al. Effect of metals on the catalytic activity of sulfated zirconia for light naphtha isomerization. Catal Surv Asia 9, 17–24 (2005). https://doi.org/10.1007/s10563-005-3333-0
Issue Date:
DOI: https://doi.org/10.1007/s10563-005-3333-0