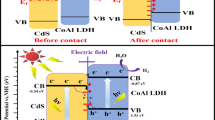
Abstract
Defect engineering is accepted as an efficient strategy for tuning the electronic structure and enhancing photocatalytic performance of layered double hydroxides (LDHs), an emerging family of two-dimensional photocatalysts, in reducing CO2 to hydrocarbons. Herein, we systematically investigate how the different types of structural defects influence electronic behavior, band edge position and Gibbs free energy barrier of CoAl-LDHs by density functional theory plus U (DFT + U) method. Our calculations indicate that CoAl-LDHs with Al defects exhibits stronger CO2 absorption capacity and narrower band gap comparing perfect CoAl-LDHs, and CoAl-LDHs with hydroxyl (–OH) defects reduce band edge down to − 1.397 V by harvesting electrons around Co atom. Furthermore, CoAl-LDHs with mixed defects of –OH and Al presents a potential synergy in photocatalytic reduction of CO2, whose calculated Gibbs free energies imply the optimal pathway for reducing CO2 to CH4 to be \({\text{CO}}_{{2}} \, \to \,*{\text{COOH}}\, \to \,*{\text{CO}}\, \to \,*{\text{CHO}}\, \to \,*{\text{CH}}_{{2}} {\text{O}}\, \to \,*{\text{CH}}_{{3}} {\text{O}}\, \to \,*{\text{CH}}_{{3}} {\text{OH}}\, \to \,*{\text{CH}}_{{3}} \, \to \,{\text{CH}}_{{4}}\) through altering the potential-determining step and lowering the Gibbs free energy barrier from 0.452 to 0.203 eV. Our achievement valuably predicts catalytic performance of CoAl-LDHs with single and mixed defects in photocatalytic CO2 reduction.
Graphical Abstract











Similar content being viewed by others
References
Yakir D (2011) Environ Res Lett 6:034007
Tasleem S, Tahir M (2021) Energy Fuels 35:9727–9746
Garbe J, Albrecht T, Levermann A, Donges JF (2020) Nature 585:538
Bodman RW, Rayner PJ, Karoly DJ (2013) Nat Clim Change 3:725–729
Chang XX, Wang T, Gong JL (2016) Energy Environ Sci 9:2177–2196
Datchi F, Giordano VM, Munsch P, Saitta AM (2009) Phys Rev Lett 103:185701
Hutchison JE, Richards RF (2015) J Thermophys Heat Transf 13:25–32
Fu JW, Jiang KX, Qiu XQ, Yu JG, Liu M (2020) Mater Today 32:222–243
Zhang BH, Jiang YZ, Gao MX, Ma TY, Sun WP, Pan HG (2020) Nano Energy 80:05504
Prabhu P, Jose V, Lee JM (2020) Adv Funct Mater 30:1910768
Zhang L, Chen LG, Xia SJ, Ge YL, Wang C, Feng HJ (2020) Int J Heat Mass Tranf 148:119025
Komiyama M, Li YJ (2004) Japanese J Appl Phys 43:4584–4587
Hatamie A, Khan A, Golabi M, Turner APF, Beni V, Mak WC, Sadollahkhani A, Alnoor H, Zargar B, Bano S, Nur O, Willander M (2015) Langmuir 31:10913–10921
Mishra M, Chun DM (2015) Appl Catal A 498:126–141
Zhukovskii YF, Piskunov S, Lisovski O, Bocharov D, Evarestov RA (2017) Isr J Chem 7:461–476
Yang J, Wang D, Han H, Li C (2013) Roles of cocatalysts in photocatalysis and photoelectocatalysis. Acc Chem Res 46:1900–1909
Gregoire B (2012) Ruby C carteret C. Cryst Growth Des 12:4324–4333
Tasleem S, Tahir M (2021) Int J Hydrogen Energy 46(40):20995–21012
Barbosa ACD, Fonseca CG, Wypych F, Leitao AA (2020) New J Chem 44:10137–10145
Liu XM, Fan X, Huang H, Lin HP, Gao JZ (2020) J Colloid Interface Sci 587:385–392
Wang XX, Yang Y, Diao LC, Tang Y, He F, Liu EZ, He CN, Shi CS, Li JJ, Sha JW, Ji SH, Zhang P, Ma LY, Zhao NQ (2018) ACS Appl Mater Interfaces 10:35145–35153
Gao R, Yan DP (2017) Nano Res 11:1883–1894
Saber O, Aljaafari A, Alomair HA, Alshoaibi A (2019) ChemistrySelect 4:4293–4300
Hao XJ, Tan L, Xu YQ, Wang ZL, Wang X, Bai S, Ning CJ, Zhao JW (2020) Zhao YF Song YF. Ind Eng Chem Res 59:3008–3015
Moraes PIR, Wypych F, Leitao AA (2019) J Phys Chem C 123:9838–9845
Han XX, Yang JZ, Han BY, Sun W, Zhao CF, Lu YX, Li Z, Ren J (2017) Int J Hydrogen Energy 42:177–192
Dou YB, Zhang ST, Pan T, Xu SM, Zhou AW, Pu M, Yan H, Han JB, Wei M, Evans DG, Duan X (2015) Adv Func Mater 25:2243–2249
Wu YJ, Gong YY, Liu JH, Chen TY, Liu Q, Zhu YT, Niu LY, Li C, Liu XJ, Sun CQ, Xu SQ (2020) J Alloy Compd 831:154723
Mosey N, Liao PL, Carter EA (2008) J Chem Phys 129:014103
Zhou F, Cococcioni M, Marianetti CA, Morgan D, Ceder G (2004) Phys Rev B 70:235121
Li PS, Wang MY, Duan XX, Zheng LR, Cheng XP, Zhang YF, Kuang Y, Ma Q, Feng ZX, Liu W, Sun XM (2019) Nat Commun 10:1711
Jiang Y, Guo JQ, Li XL, Wu G, Mu MM, Yin XH (2022) Sol Energy 231:705–715
Song YF, Tan L, Xu SM, Wang ZL, Xu YQ, Wang X, Hao XJ, Bai S, Ning CJ, Wang Y, Zhang WK, Jo YK, Hwang SJ, Cao XZ, Zheng XZ, Yan H, Zhao YF, Duan HY (2019) Angew Chem 58:11860–11867
Ouyang TW, Fan WY, Guo JQ, Zheng YN, Yin XH, Shen YL (2020) Phys Chem Chem Phys 22:10305–10313
Delley B (2020) J Chem Phys 113:7756–7764
Segall MD, Lindan PJD, Probert MJ, Pickard CJ, HasnipJ P, Clark SJ, Payne MC (2002) J Phys Condens Matter 14:2717–2744
Xu SM, Pan T, Dou YB, Yan H, Zhang ST, Ning FY, Shi WY, Wei M (2015) J Phys Chem C 119:18823–18834
Ropo M, Kokko K, Vitos L (2008) Phys Rev B 77:195445
Wang X, Wang Z, Bai Y, Tan L, Xu Y, Hao X, Wang J, Mahadi AH, Zhao Y, Zheng L, Song YF (2019) J Energy Chem 46:1–7
Xu SM, Yan H, Wei M (2017) J Phys Chem C 121:2683–2695
Wang C, Liu XM (2019) ZhangM, Geng Y, Zhao L, Li YG, Su ZM. ACS Sustain Chem Eng 7:14102–14110
Wang YL, Tian Y, Yan LK, Su ZM (2018) J Phys Chem C 122:7712–7719
Cao XM, Burch R, Hardacre C, Hu P (2011) Catal Today 165:71–79
Min Pu (2008) Liu YH, Liu LY, Dong XZ, He J, David GE. J Phys Chem Solids 69:1084–1087
Radha AV, Kamath PV, Shivakumara C (2007) J Phys Chem B 111:3411–3418
Wu CF, Liu L, Xu RP, Zhang KQ, Zhen CM, Ma L, Hou DL (2021) Mater Sci Eng B 263:114886
Li JY, Yan P, Li KL, You JJ, Wang H, Cui W, Cen WL, Chu YH, Dong F (2019) J Mater Chem A 7:17014–17021
Khan AA, Tahir M, Mohamed AR (2022) Chem Eng J 433:133693
Khan AA, Tahir M (2021) Energy Fuels 35(10):8922–8943
Ji YF, Luo Y (2016) ACS Catal 6:2018–2025
Acknowledgements
We genuinely appreciate the financial support by the National Natural Science Foundation of China (NSFC, Grant No. 21776220) and the Key Project of Tianjin Natural Science Foundation (Grant No. 19JCZDJC37600) and Training Project of Innovation Team of Colleges and Universities in Tianjin (TD13-5020) and sported by the open foundation of State Key Laboratory of Chemical Engineering (Grant No. SKL-ChE-20B05). We also acknowledge the Beijing National Supercomputing Center for providing the computational resources and Materials Studio.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, J., Shen, H., Wu, G. et al. Synergy of Various Defects in CoAl-Layered Double Hydroxides Photocatalyzed CO2 Reduction: A First-Principles Study. Catal Lett 153, 933–944 (2023). https://doi.org/10.1007/s10562-022-04038-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04038-8