Abstract
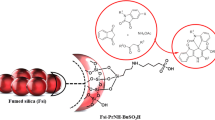
A novel silica supported ferrocene appended N-heterocyclic carbene-palladium complex (SilFemBenzNHC@Pd) has been prepared and characterized by using fourier transform infrared (FT-IR), fourier transform Raman (FT-Raman), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), thermogravimetric analysis (TGA) and energy dispersive X-ray analysis (EDX). This novel complex served as a robust heterogeneous catalyst for the synthesis of biaryls via homocoupling of aryl boronic acids under base-free conditions in water. Recyclability experiments were executed successfully for six successive runs.
Graphic Abstract











Similar content being viewed by others
References
Wanzlick HW (1962) Angew Chem Int Ed 1:75
Diez-Gonzalez S, Marion N, Nolan SP (2009) Chem Rev 109:3612
Arduengo AJ, Harlow RL, Kline M (1991) J Am Chem Soc 113:361
Fortman GC, Nolan SP (2011) Chem Soc Rev 40:5151
Huynh HV (2018) Chem Rev 118:9457
Herrmann WA, Köcher C (1997) Angew Chem 36:2162
Hans M, Lorkowski J, Demonceau A, Delaude L (2015) Beilstein J Org Chem 11:2318
Kühl O (2009) Coord Chem Rev 253:2481
Benhamou L, Chardon E, Lavigne G, Bellemin-Laponnaz S, César V (2011) Chem Rev 111:2705
Enders D, Niemeier O, Henseler A (2007) Chem Rev 107:5606
Díez-González S, Marion N, Nolan SP (2009) Chem Rev 109:3612
Herrmann WA (2002) Angew Chem Int Ed 41:1290
Hopkinson MN, Richter C, Schedler M, Glorius F (2014) Nature 510:485
Wang W, Cui L, Sun P, Shi L, Yue C, Li F (2018) Chem Rev 118:9843
Zhong R, Lindhorst AC, Groche FJ, Kühn FE (2017) Chem Rev 117:1970
Sommer WJ, Weck M (2007) Coord Chem Rev 251:860
Cazin CSJ (2009) CR Chimie 12:1173
Ye R, Zhukhovitskiy AV, Kazantsev RV, Fakra SC, Wickemeyer BB, Toste FD, Somorjai GA (2018) J Am Chem Soc 140:4144
Ranganath KVS, Onitsuka S, Kiran Kumar A, Inanaga J (2013) Catal Sci Tech 3:2161
Qin L, Ji Y, Ding T, Liu B, Wang R, Ji L, Gao G (2020) Catal Lett 150:1196
Vishal K, Fahlman BD, Sasidhar BS, Patil SA, Patil SA (2017) Catal Lett 147:900
Rafiee F, Mehdizadeh N (2018) Catal Lett 148:1345
Cabri W, Candiani I (1995) Acc Chem Res 28:2
Smith GB, Dezeny GC, Hughes DL, King AO, Verhoeven TR (1994) J Org Chem 59:8151
Hassan J, Penalva V, Lavenot L, Gozzi C, Lemaire M (1998) Tetrahedron 54:13793
Corbet J-P, Mignani G (2006) Chem Rev 106:2651
Martin R, Buhwald SL (2008) Acc Chem Res 41:1461
Karimi B, Esfahani FK (2011) Chem Commun 47:10452
Prastaro A, Ceci P, Chiancone E, Boffi A, Fabrizi G, Cacchi S (2010) Tetrahedron Lett 51:2550
Felpin F-X, Sengupta S (2019) Chem Soc Rev 48:1150
Kylmälä T, Tois J, Xu Y, Franzén R (2009) Cent Eur J Chem 7:818
Stille JK (1986) Angew Chem Int Ed Engl 25:508
Cordovilla C, Bartolomé C, Martínez-Ilarduya JM, Espinet P (2015) ACS Catal 5:3040
Ullmann F, Bielecki J (1901) J Ber Dtsch Chem Ges 34:2174
Fanta PE (1946) Chem Rev 38:139
Jiang J, Du L, Ding Y (2020) Mini-Rev Org Chem 17:26
Miyaura N, Yamada K, Suzuki A (1979) Tetrahedron Lett 20:3437
Beletskaya IP, Alonso F, Tyurin V (2019) Coord Chem Rev 385:137
Chatterjee A, Ward TR (2016) Catal Lett 146:820
Baran T (2019) Catal Lett 149(6):1721
Baran T (2019) Catal Lett 149:1496
Liu C, Xu W, Xiang D, Luo Q, Zeng S, Zheng L, Tan Y, Ouyang Y, Lin H (2020) Catal Lett 150:2558
Feizi Mohazzab B, Jaleh B, Nasrollahzadeh M, Issaabadi Z (2019) Catal Lett 149:169
Yuan S, Chang J, Yu B (2020) Topics Curr Chem 378:23
Simonetti M, Cannas DM, Larrosa I (2017). Adv Organomet Chem. 67:299. https://doi.org/10.1016/bs.adomc.2017.03.002
García-López JA, Greaney MF (2016) Chem Soc Rev 45:6766
Vasconcelos SNS, Reis JS, de Oliveira IM, Balfour MN, Stefani HA (2019) Tetrahedron 75:1865
Li M-X, Tang Y-L, Gao H, Mao Z-W (2020) Tetrahedron Lett 61:151784
Long B-F, Qin G-F, Huang Q, Xiong T, Mi Y, Hu F-L, Yin X-H (2019) J Iran Chem Soc 16:2639
Ostrowska S, Rogalski S, Lorkowski J, Walkowiak J, Pietraszuk C (2018) Synlett 29:1735
Sk MP, Jana CK, Chattopadhyay A (2013) Chem Commun 49:8235
Matsuda T, Asai T, Shiose S, Kato K (2011) Tetrahedron Lett 52:4779
Vogler T, Studer A (2008) Adv Synth Catal 350:1963
Zhao H, Mao G, Han H, Song J, Liu Y, Chu W, Sun Z (2016) RSC Adv 6:41108
Cao YN, Tian XC, Chen XX, Yun YX, Gao F, Zhou XL (2017) Synlett 28:601
Valiente A, Carrasco S, Sanz-Marco A, Tai CW, Gomez AB, Martin-Matute B (2019) Chem Cat Chem 11:3933
Ahmadi A, Sedaghat T, Azadi R, Motamedi H (2020) Catal Lett 150:112
Baran T, Sargin I, Kaya M, Menteş A (2016) J Mol Catal A Chem 420:216
Baran T, Baran NY, Menteş A (2018) Appl Organomet Chem 32(2):e4076
Baran T, Sargın I, Kaya M, Mulerčikas P, Kazlauskaitė S, Menteş A (2018) Chem Eng J 331:102
Baran T, Menteş A (2017) J Mol Struct 1134(15):591
Baran T (2018) J Macromol Sci A 55(3):280
Anjali JK, Sreekumar K (2019) Catal Lett 149:1952
Labattut A, Fayssal SA, Buendia J, Abdellah I, Huc V, Martini C, Schulz E (2020) React Chem Eng 5:1509. https://doi.org/10.1039/D0RE00118J
Boztepe C, Künkül A, Gürbüz N (2020) J Mol Struct 1209:127948
Tamami B, Farjadian F, Ghasemi S, Allahyari H (2013) New J Chem 37:2011
Kandathil V, Kulkarni B, Siddiqa A, Kempasiddaiah M, Sasidhar BS, Patil SA, Patil SA (2020) Catal Lett 150:384
Mizusaki T, Matsumoto K, Takeuchi K, Fukaya N, Takagi Y, Choi J-C (2019) Organometallics 38(9):1872
Lei Y, Lan G, Fan M, Li G (2020) Catal Commun 140:106007
Majeed MH, Shayesteh P, Wallenberg LR, Persson AR, Johansson N, Ye L, Schnadt J, Wendt OF (2017) Chem Eur J 23(35):8457
Kurane R, Jadhav J, Khanapure S, Salunkhe R, Rashinkar G (2013) Green Chem 15:1849
Gajare S, Patil A, Kale D, Bansode P, Patil P, Rashinkar G (2020) Catal Lett 150:243
Gao Y, Twamley B, Shreeve JM (2004) Inorg Chem 43:3406
Adamo C, Amatore C, Ciofini I, Jutand A, Lakmini H (2006) J Am Chem Soc 128:6829
Cavallo L, Correa A, Costabile C, Jacobsen H (2005) J Organomet Chem 690:5407
Barbaro P, Bianchini C, Giambastiani G, Parisel SL (2004) Coord Chem Rev 248:2131
Ferrocenes: Homogeneous Catalysis, Organic Synthesis, Material Science, eds. A. Togni and T. Hayashi (Wiley-VCH, Weinheim, 1995).
Siemeling U, Auch TC (2005) Chem Soc Rev 34:584
Metallocenes, eds. A. Togni and R.L. Halterman (Wiley-VCH, Weinheim, 1998).
Atkinson RCJ, Gibson VC, Long NJ (2004) Chem Soc Rev 33:313
Cheng K, Xin B, Zhang Y (2007) J Mol Catal A: Chem 273:240
Wong MS, Zhang XL (2001) Tetrahedron Lett 42:4087
Punna S, Diaz DD, Finn MG (2004) Synlett 13:2351
Kabalka GW, Wang L (2002) Tetrahedron Lett 43:3067
Klingensmith LM, Leadbeater NE (2003) Tetrahedron Lett 44:765
Lei A, Zhang X (2002) Tetrahedron Lett 43:2525
Dwivedi S, Bardhan S, Ghosh P, Das S (2014) RSC Adv 4:41045
Acknowledgement
We gratefully acknowledge Indian Institute of Technology, Madras (IITM) and Indian Institute of Sciences (IISc), Bangalore for providing spectral facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khanapure, S., Pore, D., Jagadale, M. et al. Sustainable Synthesis of Biaryls Using Silica Supported Ferrocene Appended N-Heterocyclic Carbene-Palladium Complex. Catal Lett 151, 2237–2249 (2021). https://doi.org/10.1007/s10562-020-03480-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03480-w