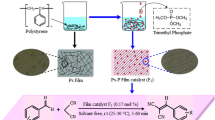
Abstract
Polystyrene nanometer-sized particles supported alkaline 1-propyl imidazolium ionic liquids catalysts (nano-PS-CH2-[pIM][B]) were prepared and characterized by FT-IR, SEM, TG/DTA, BET and particle size distribution analysis. The results suggested that ionic liquids were successfully loaded on the surface of polymer carriers by covalent bond with high alkali amount. It showed excellent catalytic activities for Knoevenagel condensation especially in aqueous phase, which much higher than that of NaOH and micro-PS-CH2-[pIM][B]. It will be expected for the potential application of organic reactions in aqueous phase.
Graphical Abstract









Similar content being viewed by others
References
Wilkes JS (2004) J Mol Catal A 214:11
Ni B, Headley AD (2010) Chemistry 16:4426
Lee JW, Shin JY, Chun YS (2010) Acc Chem Res 43:985
Qiao K, Deng Y (2001) J Mol Catal A 171:81
Mehnert CP, Mozeleski EJ, Cook RA (2003) Chem Commun 34:3010
Riisager A, Jørgensen B, Wasserscheid P (2006) Chem Commun 37:994
Liu S, Lu LI, Shitao YU (2010) Chin J Catal 31:1433
Durand J, Teuma E, Gómez M (2007) Cr Chim 10:152
Mehnert CP, Cook RA, Dispenziere NC (2002) J Am Chem Soc 124:12932
Zhu A, Jiang T, Han B, Zhang J, Xie Y, Ma X (2007) Green Chem 38:169
Shao Y, Wan H, Miao J, Guan G (2013) React Kinet Mech Cat 109:149
Wu X, Wang M, Xie Y, Chen C, Li K (2016) Appl Catal A 519:146
Lin Y, Wang F, Zhang Z, Yang J, Wei Y (2014) Fuel 116:273
Movahed SK, Esmatpoursalmani R, Bazgir A (2014) RSC Adv 4:14586
Rodríguezpérez L, Teuma E, Falqui A, Gómez M, Serp P (2008) Chem Commun 35:4201
Yuan H, Jiao QZ, Zhang Y, Zhang J, Wu Q (2016) Catal Lett 146:1
Dr CPM (2004) Chem Eur J 11:50
Zhang Q, Zhang S, Deng Y (2011) Green Chem 13:2619
Patra T, Karmakar S, Upadhyayula S (2017) Tetrahedron Lett 58:1531
Valkenberg MH, Decastro C, Hölderich WF (2001) Top Catal 14:139
Qiu XH, Xie Y, Wang XY et al (2013) Asian J Chem 25:1673
Kumar P, Vermeiren W, Dath JP, Hoelderich WF (2006) Appl Catal A 304:131
Wang G, Yu N, Lin P et al (2008) Catal Lett 123:252
Dötterl M, Helmut G (2012) Chemcatchem 4:660
Kim JH, Kim JW, Shokouhimehr A, Lee YS (2005) J Org Chem 70:6714
Karbass N, Sans V, Garcia-Verdugo E, Burguete MI, Luis SV (2006) Chem Commun (29:):3095
Itoh H, Naka K, Chujo Y (2004) J Am Chem Soc 126:3026
Huang J, Jiang T, Gao H, Han B, Liu Z, Wu W, Chang Y, Zhao G (2004) Angew Chem Int Ed 43:1397
Kume Y, Qiao K, Tomida D, Yokoyama C (2008) Catal Commun 9:369
He X, Liu Z, Fan F, Qiang S, Cheng L, Yang W (2015) J Nanopart Res 17:1
Wang T, Wang W, Yuan L, Chen X, Li C, Zhang Y (2017) RSC Adv 7:2836
Burguete MI, Erythropel H, Garcia-Verdugo E, Luis SV, Sans V (2008) Green Chem 10:401
Sugimura R, Qiao K, Tomida D, Yokoyama C (2007) Catal Commun 8:770
Xu Z, Wan H, Miao J, Han M, Yang C, Guan G (2010) J Mol Catal A 332:152
Landfester K, Bechthold N, Franca Tiarks A, Antonietti M (1999) Macromolecules 32:5222
Liu QQ, Li W, Yu HJ, Tan QH (2008) Eur Polym J 44:2516
Quiñones R, Gawalt ES (2008) Langmuir 24:10858
Schork FJ, Luo Y, Smulders W, Russum JP, Butté A, Fontenot K (2005) Adv Polym Sci 175:129
Baruch-Sharon S, Margel S (2010) Colloid Polym Sci 288:869
Shlomo M, Eliahu N, Inna F (1991) J Polym Sci Pol Chem 29:347
Davankov VA, Pastukhov AV, Tsyurupa M (2000) Polym Sci 38:1553
Acknowledgements
We are very grateful to the National Natural Science Foundation of China (NNSFC) for the support (No. 21576025).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ma, M., Li, H., Yang, W. et al. Polystyrene Nanometer-Sized Particles Supported Alkaline Imidazolium Ionic Liquids as Reusable and Efficient Catalysts for the Knoevenagel Condensation in Aqueous Phase. Catal Lett 148, 134–143 (2018). https://doi.org/10.1007/s10562-017-2255-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2255-6