Abstract
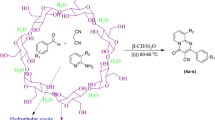
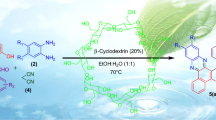
An efficient and green method has been developed for the synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones derivatives by employing 20 mol% β-cyclodextrin via a one-pot multicomponent reaction of aldehydes, malononitrile and phthalhydrazide in H2O–EtOH (4:1) at 100 °C for first time under neutral condition. The catalyst could be recovered and reused for four consecutive cycles without appreciable loss in catalytic activity.
Graphical Abstract








Similar content being viewed by others
References
Al-Assar F, Zelenin KN, Lesiovskaya EE, Bezhan IP, Chahchir BA (2002) Pharma Chem J 36:598–603
Jain RP, Vederas JC (2004) Bioorg Med Chem Lett 14:3655–3658
Carling RW, Moore KW, Street LJ, Wild D, Isted C, Lesson PD, Thomas SO, cooner D, Mckernan RM, Quirk K, Cook SM, Atach JR, Waftord KA, Thompson SA, Dawson GR, Ferris P, Castro JL (2004) J Med Chem 47:1807–1822
Grasso S, DeSarro G, Micale N, Zappala M, Puia G, Baraldi M, Demicheli C, (2000) J Med Chem 43:2851–2859
Nomoto Y, Obase H, Takai H, Teranishi M, Nakamura J, Kubo K (1990) Chem Pharm Bull 38:2179–2183
Watanabe N, Kabasawa Y, Takase Y, Matsukura M, Miyazaki K, Ishihara H, Kodama K, Adachi H (1998) J Med Chem 41:3367–3372
Chebanov VA, Muravyova EA, Desenko SM, Musatov VI, Knyazeva IV, Shishkina SV, Shishkina OV, Kappe CO (2006) J Comb Chem 8:427–434
Dondoni A, Massi A, Sabbatini S, Bertolasi V (2002) J Org Chem 67:6979–6994
Liu JN, Li J, Zhang L, Song LP, Zhang M, Cao WG, Zhu SZ, Deng HG, Shao M (2012)Tetrahedron Lett 53:2469–2472
Kim JS, Rhee HK, Park HJ, Lee SK, Lee CO, Park Choo H-Y (2008) Bioorg Med Chem 16:4545–4550
El-Sakka SS, Soliman AH, Imam AM (2009) Afinidad 66:167
Ryu CK, Park RE, Ma MY, Nho JH (2007) Bioorg Med Chem Lett 17:2577–2580
Li J, Zhao YF, Yuan XY, Xu JX, Gong P (2006) Molecules 11:574–582
Ghahremanzadeh R, Shakibaei GI, Bazgir A (2008) Synlett 8:1129–1132
Mohamadpour F, Maghsoodlou MT, Heydari R, Lashkari M (2016) Res Chem Intermed 42:7841–7853
Bashti A, Ali Reza Kiasat AR, Mokhtari B (2015) RSC Adv 5:25816–25823
Nabid MR, Tabatabaie SJ, Gahremanzadeh R, Bazgir A (2010) Ultrason Sonochem 17:159–161
Raghuvanshi DS, Singh KN (2011) Tetrahedron Lett 52:5702–5705
Safaei-Ghomi J, Shahbazi-Alavi H, Ziarati A, Teymuri R, Saberi MR (2014) Chin Chem Lett 25:401–405
Reddy MV, Tae Jeong Y (2013) Tetrahedron Lett 54:3546–3549
Shaterian HR, Mohammadnia M (2014) Res Chem Intermed 40:371–383
Shi-Hua Song, Jun Zhong, Yan-Hong He, Zhi Guan (2012) Tetrahedron Lett 53:7075–7077
Ghorbani-Vaghei R, Noori S, Toghraei-Semiromi Z, Salimi Z (2014) RSC Adv 4:47925–47928
Karthikeyan G, Pandurangan A (2012) J Mol Catal A 361–362:58–67
Maleki B, Nam Chalaki SB, Ashrafi SS, Seresht ER, Moeinpourb F, Khojastehnezhad A, Tayebee R (2015) Appl Organometal Chem 29:290–295
Mulik AG, Chandam DR, Patil DR, Patil PP, Mulik GN, Salunkhe ST, Deshmukh MB (2015) Res Chem Intermed 41:10085–10096
Vafaee A, Davoodnia A, Pordel M, Bozorgmehr MR (2015) Oriental J Chem 31(4):2153–2158
Kidwai M, Chauhan R (2014) J Hetero Chem 51:1689–1696
Chaskar A (2014) Curr Catal 3(3):266–273
Breslowong R, Dong SD (1998) Chem Rev 98:1997–2011
Desper JM, Breslow R (1994) J Am Chem Soc 116:12081–12082
Szejtli J (1998) Chem Rev 98:1743–1754
Rai P, Srivastava M, Yadav S, Singh J, Jagdamba S (2015) Catal Lett 145:2020–2028
Kumar A, Tripathi VD, Kumar P (2011) Green Chem 13:51–54
Kumar A, Shukla RD (2015) Green Chem 17:848–851
Kumar S, Ahmed N (2016) Green Chem 18:648–656
Azath IA, Puthiaraj P, Pitchumani K (2013) ACS Sustain Chem Eng 1:174–179
Sukumari S, Azath IA, Pitchumani K (2012) Synlett 23:2328–2332
Kanagaraj K, Pitchumani K (2013) J Org Chem 78:744–751
Londhe BS, Pratap UR, Mali JR, Mane RA (2010) Bull Korean Chem Soc 31(8):2329–2332
Tayade YA, Patil DR, Wagh YB, Jangle AD, Dalal DS (2015) Tetrahedron Lett 56:666–673
Patil DR, Wagh YB, Ingole PG, Singh K, Dalal DS (2013) New J Chem 37:3261–3266
Deshmukh MB, Wagh ND, Sikder AK, Borse AU, Dalal DS 1014 Ind Eng Chem Res 53:19375–19379
Patil DR, Dalal DS (2012) Chin Chem Lett 23:1125–1128
Patil DR, Ingole PG, Singh K, Dalal DS (2013) J Incl Phenom Macro Chem 76:327–332
Wagh YB, Kuwar A, Patil DR, Tayade YA, Jangale AD, Terdale SS, Trivedi DR, Gallucci J, Dalal DS (2015) Ind Eng Chem Res 54:9675–9682
Wagh YB, Tayade YA, Padvi SA, Patil BS, Patil NB, Dalal DS (2015) Chin Chem Lett 26:1273–1277
Jangale AD, Kumavat PP, Wagh YB, Tayade YA, Mahulikar PP, Dalal DS (2015) Synth Commun 45:376–385
Dalal KS, Tayade YA, Wagh YB, Trivedi DR, Dalal DS, Chaudhari BL (2016) RSC Adv 6:14868–14879
Padvi SA, Tayade YA, Wagh YB, Dalal DS (2016) Chin Chem Lett 27:714–720
Tayade YA, Padvi SA, Wagh YB, Dalal DS (2015) Tetrahedron Lett 56:2441–2447
Kokkirala S, Murthy SN, Venkata D, Nageswar YVD (2011) Eur J Chem 2:272–275
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tayade, Y.A., Dalal, D.S. β-Cyclodextrin as a Supramolecular Catalyst for the Synthesis of 1H-Pyrazolo[1,2-b]phthalazine-5,10-dione Derivatives in Water. Catal Lett 147, 1411–1421 (2017). https://doi.org/10.1007/s10562-017-2032-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2032-6