Abstract
Several acidic ionic liquids (ILs) with different cations and anions were used as catalysts in the transesterification of methyl acetate with n-butanol. The IL [omim][PF6] can enhance the catalytic activity of the partially soluble IL catalyst. The kinetics for the transesterification using the catalysts [HSO3bmim][p-TS] and [HSO3bmim][OTF] were measured. The ideal homogeneous model and the nonideal homogeneous (NIH) model were used to correlate the kinetic data. The NIH model was more reliable to describe the reaction rate.
Graphical Abstract









Similar content being viewed by others
Abbreviations
- IL:
-
Ionic liquid
- MRD:
-
Mean relative deviation
- BuOH:
-
n-Butanol
- MeOAc:
-
Methyl acetate
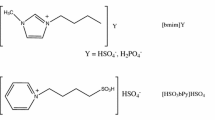
- [HSO3bmim][Cl]:
-
1-Sulfobutyl-3-methylimidazolium chloride
- [HSO3bmim][OTF]:
-
1-Sulfobutyl-3-methylimidazolium trifluoromethanesulfonate
- [HSO3bmim][p-TS]:
-
1-Sulfobutyl -3-methylimidazolium tosylate
- [HSO3bpy][OTF]:
-
N-sulfobutylpyridinium trifluoromethanesulfonate
- [HSO3bHim][HSO4]:
-
1-Sulfobutylimidazolium hydrogensulfate
- [omim][PF6]:
-
1-Octyl-3-methylimidazolium hexafluorophosphate
- r 0 :
-
Initial reaction rate (mol/(L min))
- r :
-
Reaction rate (mol/(L min))
- c :
-
Mole concentration (mol/L)
- E :
-
Constant defined in Eq 2
- c cat :
-
Mole concentration of ionic liquid catalysts (mol/L)
- k + :
-
Forward reaction rate constant (L2/(min mol2))
- k - :
-
Backward reaction rate constant (L2/(min mol2))
- \( k_{ + }^{0} \) :
-
Arrhenius pre-exponential factors (L2/(min mol2))
- \( k_{ - }^{0} \) :
-
Arrhenius pre-exponential factors (L2/(min mol2))
- E A+ :
-
Activation energy of forward reaction (kJ/mol)
- E A− :
-
Activation energy of backward reaction (kJ/mol)
- V :
-
Volume of the reaction mixture (L)
- R :
-
General gas constant, 8.314 kJ/(mol K)
- N :
-
Total number of experimental data
- v :
-
Stoichiometric coefficient
- x :
-
Mole fraction
- ρ:
-
Molar density of reaction mixture (mol/L)
- t :
-
Time (min)
- α:
-
Activity
- γ:
-
Activity coefficient
- 0:
-
Standard state/conditions
- +:
-
Forward reaction
- −:
-
Backward reaction
- cal :
-
Catalyst
- calc :
-
Calculated value
- exp :
-
Experimental value
References
Grasa GA, Guveli T, Singh R, Nolan SP (2003) J Org Chem 68:2812–2819
Kim HJ, Kang BS, Kim MJ, Park YM, Kim DK, Lee JS, Lee KY (2004) Catal Today 93–95:315–320
Pan FY, Wang SP, Ma XB, Li ZH, Xu GH (2004) Chem Ind Eng 21:174–176
Luyben WL (2010) Ind Eng Chem Res 50:1247–1263
Steinigeweg S, Gmehling J (2004) Chem Eng Process 43:447–456
Wang QQ, Wu XL (2011) Sichuan Huagong 14:15–17
Jimenez L, Garvin A, Costa-Lopez J (2002) Ind Eng Chem Res 41:6735–6744
Sert E, Atalay FS (2012) Ind Eng Chem Res 51:6350–6355
Sharma YC, Singh B, Korstad J (2011) Biofuels Bioprod Biorefin 5:69–92
Palani A, Gokulakrishnan N, Palanichamy M, Pandurangan A (2006) Appl Catal A Gen 304:152–158
Tao DJ, Lu XM, Lu JF, Huang K, Zhou Z, Wu YT (2011) Chem Eng J 171:1333–1339
Welton T (1999) Chem Rev 99:2071–2083
Hallett JP, Welton T (2011) Chem Rev 111:3508–3576
Domingos JB, Dupont J (2007) Catal Commun 8:1383–1385
Kotadia DA, Soni SS (2013) Monatsh Chem 144:1735–1741
de los Rios AP, Hernandez-Fernandez FJ, Rubio M, Gomez D, Villora G (2010) Desalination 250:101–104
Qureshi ZS, Deshmukh KM, Bhor MD, Bhanage BM (2009) Catal Commun 10:833–837
Kuschnerow JC, Titze-Frech K, Schulz PS, Wasserscheid P, Scholl S (2013) Chem Eng Technol 36:1643–1650
Peng YM, Cui XB, Zhang Y, Feng TY, Tian Z, Xue LX (2013) Appl Catal A Gen 466:131–136
Peng YM, Cui XB, Zhang Y, Feng TY, Tian Z, Xue LX (2014) Int J Chem Kinet 46:116–125
Wang HX, Wu CM, Bu XW, Tang WL, Li L, Qiu T (2014) Chem Eng J 246:366–372
Bozek-Winkler E, Gmehling J (2006) Ind Eng Chem Res 45:6648–6654
Jimenez L, Garvin A, Costa-Lopez J (2002) Ind Eng Chem Res 41:6663–6669
Cui XB, Cai JL, Zhang Y, Li R, Feng TY (2011) Ind Eng Chem Res 50:11521–11527
Thornazeau C, Olivier-Bourbigou H, Magna L, Luts S, Gilbert B (2003) J Am Chem Soc 125:5264–5265
Zhao YW, Long JX, Deng FG, Liu XF, Li Z, Xia CG, Peng JJ (2009) Catal Commun 10:7323–7736
Elavarasan P, Kishore KVK, Upadhyayuala S (2009) Bull Catal Soc India 8:107–113
Rodriguez AV, Plesu V, Tarragona AC, Ruiz JB, Ruiz AEB, Llacuna JL (2013) Chem Eng Trans 35:1093–1098
Sanz MT, Murga R, Beltran S, Cabezas JL, Coca J (2002) Ind Eng Chem Res 41:512–517
Choi JL, Hong WH, Chang HN (1996) Int J Chem Kinet 28:37–41
Acknowledgments
Financial support from the Innovation Fund of Tianjin University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Z., Cui, X., Yu, X. et al. Transesterification of Methyl Acetate with n-Butanol Catalyzed by Single and Mixed Ionic Liquids. Catal Lett 145, 1281–1289 (2015). https://doi.org/10.1007/s10562-015-1506-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-015-1506-7