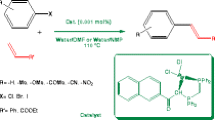
Abstract
An air-stable tetraphosphine N,N,N′,N′-tetra(diphenylphosphinomethyl)-benzene-1,3-diamine (L3) was easily prepared in two steps from triphenylphosphine, which in combination with [Pd(η3-C3H5)Cl]2 affords an efficient catalyst for Suzuki–Miyaura coupling of activated chloroarenes. Even at high temperature of 130 °C, this catalyst exhibits good stability and longevity, and could allow a high turnover number of 680,000 to be reached.
Graphical Abstract

.


Similar content being viewed by others
References
Miyaura N, Suzuki A (1995) Chem Rev 95:2457
Suzuki A (2011) Angew Chem Int Ed 50:6722
Capdeville R, Buchdunger E, Zimmermann J, Matter A (2002) Nat Rev Drug Discov 1:493
Vilaró M, Arsequell G, Valencia G, Ballesteros A, Barluenga J (2008) Org Lett 10:3243
Pomeisl K, Holý A, Pohl R, Horská K (2009) Tetrahedron 65:8486
Heiskanen JP, Hormi OEO (2009) Tetrahedron 65:518
Mohanty S, Suresh D, Balakrishna MS, Mague JT (2008) Tetrahedron 64:240
Littke AF, Dai C-Y, Fu GC (2000) J Am Chem Soc 122:4020
Kataoka N, Shelby Q, Stambuli JP, Hartwig JF (2002) J Org Chem 67:5553
Fleckenstein CA, Plenio H (2007) Chem Eur J 13:2701
Dawood KM (2007) Tetrahedron 63:9642
Han W, Liu C, Jin Z-L (2008) Adv Synth Catal 350:501
Jin M-J, Lee D-H (2010) Angew Chem Int Ed 49:1119
Yamada YMA, Sarkar SM, Uozumi Y (2012) J Am Chem Soc 134:3190
Zapf A, Ehrentraut A, Beller M (2000) Angew Chem Int Ed 39:4153
Glegoła K, Framery E, Pietrusiewicz KM, Sinou D (2006) Adv Synth Catal 348:1728
Xu C, Gong J-F, Guo T, Zhang Y-H, Wu Y-J (2008) J Mol Catal A 279:69
Wong SM, So CM, Chung KH, Lau CP, Kwong FY (2012) Eur J Org Chem 22:4172–4177
Marion N, Nolan SP (2008) Acc Chem Res 41:1440
Karimi B, Akhavan PF (2011) Inorg Chem 50:6063
Wolfe JP, Buchwald SL (1999) Angew Chem Int Ed 38:2413
Wolfe JP, Singer RA, Yang BH, Buchwald SL (1999) J Am Chem Soc 121:9550
Yin J–J, Rainka MP, Zhang X–X, Buchwald SL (2002) J Am Chem Soc 124:1162
Walker SD, Barder TE, Martinelli JR, Buchwald SL (2004) Angew Chem Int Ed 43:1871
Barder TE, Walker SD, Martinelli JR, Buchwald SL (2005) J Am Chem Soc 127:4685
Martin R, Buchwald SL (2008) Acc Chem Res 41:1461
Bedford RB, Hazelwood SL, Limmert ME (2002) Chem Commun 22:2610
Laurenti D, Feuerstein M, Pèpe G, Doucet H, Santelli M (2001) J Org Chem 66:1633
Feuerstein M, Doucet H, Santelli M (2001) Synlett 9:1458
Feuerstein M, Laurenti D, Bougeant C, Doucet H, Santelli M (2001) Chem Commun 4:325
Doucet H, Santelli M (2006) Synlett 13:2001
Kondolff I, Doucet H, Santelli M (2007) J Mol Catal A 269:110
Stössel P, Heins W, Mayer HA, Fawzi R, Steimann M (1996) Organometallics 15:3393
Schill H, de Meijere A, Yufit DS (2007) Org Lett 9:2617
Hierso J-C, Fihri A, Amardeil R, Meunier P, Doucet H, Santelli M, Donnadieu B (2003) Organometallics 22:4490
Hierso J-C, Smaliy R, Amardeil R, Meunier P (2007) Chem Soc Rev 36:1754
Roy D, Mom S, Beaupérin M, Doucet H, Hierso J-C (2010) Angew Chem Int Ed 49:6650
Monnereau L, Sémeril D, Matt D, Toupet L (2010) Chem Eur J 16:9237
Zaborova E, Deschamp J, Guieu S, Blériot Y, Poli G, Ménand M, Madec D, Prestat G, Sollogoub M (2011) Chem Commun 47:9206
Wang K, Fu Q, Zhou R, Zheng X-L, Fu H-Y, Chen H, Li R-X (2013) Appl Organomet Chem 27:232
Wang K, Yi T, Yu X-J, Zheng X-L, Fu H-Y, Chen H, Li R-X (2012) Appl Organomet Chem 26:342
Zhang Y, Yi T, Wang K, Fu H-Y, Chen H, Li R-X (2012) Chin J Org Chem 32:790
Balch AL, Olmstead MM, Rowley SP (1990) Inorg Chim Acta 168:255
Fawcett J, Hoye PAT, Kemmitt RDW (1993) J Chem Soc Dalton Trans: 2563
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21202104).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, K., Wang, W., Luo, H. et al. An Easily Prepared Tetraphosphine and Its Use in the Palladium-Catalyzed Suzuki–Miyaura Coupling of Aryl Chlorides. Catal Lett 143, 1214–1219 (2013). https://doi.org/10.1007/s10562-013-1028-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-013-1028-0