Abstract
In the present study, the nickel-catalyzed cross coupling of aryl halides with benzyl zinc bromides or dialkyl zinc reagents to create C(sp 2)–C(sp 3) bonds has been explored. As pre-catalyst the well-defined and easy-accessible (bispidine)Ni(NO3)2 complex has been applied. After investigation of different reaction parameters a broad scope of C(sp 2)–C(sp 3) bond formations were feasible under mild reaction conditions.
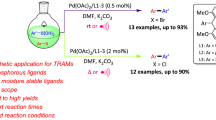
Graphical Abstract

.







Similar content being viewed by others
References
Corey EJ, Cheng XM (1989) The logic of chemical synthesis. Wiley, New York, pp 1–91
Suzuki A (2011) Angew Chem 123:6854–6869, Angew Chem Int Ed 2011, 50: 6722–6737
Negishi E (2011) Angew Chem Int Ed 50:6738–6764
de Meijere F, Diederich (eds) (2004) Metal-catalyzed cross-coupling reactions. Wiley-VCH, Weinheim
Negishi E (ed) (2002) Handbook of organopalladium chemistry for organic synthesis. Wiley-InterScience, New York
Diederich F, Stang PJ (eds) (1998) Metal-catalyzed cross-coupling reactions. Wiley-VCH, Weinhein
Jana R, Pathak TP, Sigman MS (2011) Chem Rev 111:1417–1492
Hu X (2011) Chem Sci 2:1867–1886
Rilatt RFW, Jackson J (2008) Org Chem 73:8694–8704
Zhou S, Fu GC (2003) J Am Chem Soc 125:14726–14727
Phapale VB, Buñuel E, García-Iglesias M, Cárdenas DJ (2007) Angew Chem Int Ed 46:8790–8795
Smith SW, Fu GC (2008) Angew Chem 120: 9474–9476, Angew Chem Int Ed 2008, 47:9334–9336
Jones GD, Martin JL, McFarland C, Allen OR, Hall RE, Haley AD, Brandon RJ, Konovalova T, Desrochers PJ, Pulay P, Vicic DA (2006) J Am Chem Soc 128:13175–13183
Joshi-Pangu A, Ganesh M, Biscoe MR (2011) Org Lett 13:1218–1221
Comba P, Kanellakopulos B, Katsichtis C, Lienke A, Pritzkow H, Rominger F (1998) J Chem Soc Dalton Trans 23:3997–4001
Börzel H, Comba P, Katsichtis C, Kiefer W, Lienke A, Nagel V, Pritzkow H (1999) Chem Eur J 5:1716–1721
Börzel H, Comba P, Hagen KS, Katsichtis C, Pritzkow H (2000) Chem Eur J 6:914–919
Börzel H, Comba P, Pritzkow H (2001) Chem Commun 97–98
Comba P, Kerscher M, Merz M, Müller V, Pritzkow H, Remenyi R, Schiek W, Xiong Y (2002) Chem Eur J 8:5750–5760
Börzel H, Comba P, Hagen KS, Kerscher M, Pritzkow H, Schatz M, Schindler S, Walter O (2002) Inorg Chem 41:5440–5452
Börzel H, Comba P, Hagen KS, Lampeka YD, Lienke A, Linti G, Merz M, Pritzkow H, Tsymbal LV (2002) Inorg Chim Acta 337:407–419
Comba P, Hauser A, Kerscher M, Pritzkow H (2003) Angew Chem Int Ed 42:4536–4540
Comba P, de Laorden CL, Pritzkow H (2005) Helv Chim Acta 88:647–664
Bautz J, Comba P, Que L Jr (2006) Inorg Chem 45:7077–7082
Anastasi AE, Lienke A, Comba P, Rohwer H, McGrady JE (2007) Eur J Inorg Chem 65–73
Born K, Comba P, Ferrari R, Lawrance GA, Wadepohl H (2007) Inorg Chem 46:458–464
Comba P, Kuwata S, Linti G, Tarnai M, Wadepohl H (2007) Eur J Inorg Chem 657–664
Anastasi AE, Comba P, McGrady J, Lienke A, Rohwer H (2007) Inorg Chem 46:6420–6426
Bentz A, Comba P, Deeth RJ, Kerscher M, Seibold B, Wadepohl H (2008) Inorg Chem 47:9518–9527
Born K, Comba P, Kerscher M, Linti G, Pritzkow H, Rohwer H (2009) Dalton Trans 362–367
Comba P, Daumann L, Lefebvre J, Linti G, Martin B, Straub J, Zessin T (2009) Aust J Chem 62:1238–1245
Black DSC, Deacon GB, Rose M (1995) Tetrahedron 51:2055–2076
Barnes NA, Brooker AT, Godfrey SM, Mallender PR, Pritchard RG, Sadler M (2008) Eur J Org Chem 1019–1030
Walther M, Matterna M, Juran S, Fähnemann S, Stephan H, Kraus W, Emmerling F (2011) Z Naturforsch 66b:721–728
Bleiholder C, Börzel H, Comba P, Ferrari R, Heydt M, Kerscher M, Kuwata S, Laurenczy G, Lawrance GA, Lienke A, Martin B, Merz M, Nuber B, Pritzkow H (2005) Inorg Chem 44:8145–8155
Comba P, Haaf C, Wadepohl H (2009) Inorg Chem 48:6604–6614
Atanasov M, Busche C, Comba P, El Hallak F, Martin B, Rajaraman G, van Slageren J, Wadepohl H (2008) Inorg Chem 47:8112–8125
Comba P, Merz M, Pritzkow H (2003) Eur J Inorg Chem 1711–1718
Born K, Comba P, Daubinet A, Fuchs A, Wadepohl H (2007) J Biol Inorg Chem 12:36–48
Comba P, Lang C, de Laorden CL, Muruganantham A, Rajaraman G, Wadepohl H, Zajaczkowski M (2008) Chem Eur J 14:5313–5328
Bukowski MR, Comba P, Lienke A, Limberg C, de Laorden CL, Mas-Ballesté R, Merz M, Que L Jr (2006) Angew Chem Int Ed 45:3446–3449
Someya CI, Inoue S, Krackl S, Irran E, Enthaler S (2012) Eur J Inorg Chem 1269–1277
Sammhammer A, Holzgrabe U, Haller R (1989) Arch Pharm 322:551–555
Holzgrabe U, Erciyas E (1992) Arch Pharm 325:657–663
Comba P, Nuber B, Ramlow A (1997) J Chem Soc Dalton Trans 3:347–352
Alacid E, Nájera C (2008) Org Lett 10:5011–5014
Sun G, Wang Z (2008) Tetrahedron Lett 49:4929–4932
Burns MJ, Fairlamb IJS, Kapdi AR, Sehnal P, Taylor RJK (2007) Org Lett 26:5397–5400
Amatore M, Gosmini C (2008) Chem Commun 5019–5021
Cho C-H, Sun M, Seo Y-S, Kim C-B, Park K (2005) J Org Chem 70:1482–1485
Wu GG, Chen FX, LaFrance D, Liu Z, Greene SG, Wong Y-S, Xie J (2011) Org Lett 13:5220–5223
Blachut D, Szawkalo J, Czarnocki Z (2011) Synthesis 21:3496–3506
Smit C, Fraaije MW, Minnaard AJ (2008) J Org Chem 73:9482–9485
Rubottom GM, Evain EJ (1990) Tetrahedron 46:5055–5064
Kutney JP, Cable J, Gladstone WAF, Hanssen HW, Nair GV, Torupka EJ, Warnock WDC (1975) Can J Chem 53:1796–1817
Kato S, Kurata T, Ishiguro S, Fujimaki M (1973) Agric Biol Chem 37:1759–1761
Sheldrick GM (1997) SHELXL93, Program for the refinement of crystal structures. University of Göttingen, Göttingen
Acknowledgements
Financial support from the Cluster of Excellence “Unifying Concepts in Catalysis” (funded by the Deutsche Forschungsgemeinschaft and administered by the Technische Universität Berlin) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haberberger, M., Someya, C.I., Company, A. et al. Application of a Nickel-Bispidine Complex as Pre-Catalyst for C(sp 2)–C(sp 3) Bond Formations. Catal Lett 142, 557–565 (2012). https://doi.org/10.1007/s10562-012-0806-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-012-0806-4