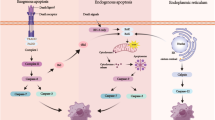
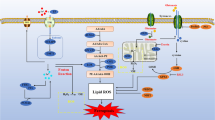
Abstract
Myocardial ischemia reperfusion injury (MIRI) represents a prevalent and severe cardiovascular condition that arises primarily after myocardial infarction recanalization, cardiopulmonary bypass surgery, and both stable and unstable angina pectoris. MIRI can induce malignant arrhythmias and heart failure, thereby increasing the morbidity and mortality rates associated with cardiovascular diseases. Hence, it is important to assess the potential pathological mechanisms of MIRI and develop effective treatments. The role of circular RNAs (circRNAs) in MIRI has increasingly become a topic of interest in recent years. Moreover, significant evidence suggests that circRNAs play a critical role in MIRI pathogenesis, thereby representing a promising therapeutic target. This review aimed to provide a comprehensive overview of the current understanding of the role of circRNAs in MIRI and discuss the mechanisms through which circRNAs contribute to MIRI development and progression, including their effects on apoptosis, inflammation, oxidative stress, and autophagy. Furthermore, the potential therapeutic applications of circRNAs in MIRI treatment, including the use of circRNA-based therapies and modulation of circRNA expression levels, have been explored. Overall, this paper highlights the importance of circRNAs in MIRI and underscores their potential as novel therapeutic targets.

Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Becker AC, Lantz CW, Forbess JM, et al. Cardiopulmonary bypass-induced inflammation and myocardial ischemia and reperfusion injury stimulates accumulation of soluble MER. Pediatr Crit Care Med. 2021;22(9):822–31. https://doi.org/10.1097/PCC.0000000000002725
Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the american heart association. Circulation. 2022;145(8):e153-e639. https://doi.org/10.1161/CIR.0000000000001052
Zhang D, Wu H, Liu D, et al. Research progress on the mechanism and treatment of inflammatory response in myocardial ischemia-reperfusion injury. Heart Surg Forum. 2022;25(3):E462–8. https://doi.org/10.1532/hsf.4725
Su Y, Zhu C, Wang B, et al. Circular RNA Foxo3 in cardiac ischemia-reperfusion injury in heart transplantation: a new regulator and target. Am J Transplant. 2021;21(9):2992–3004. https://doi.org/10.1111/ajt.16475
Sánchez-Hernández CD, Torres-Alarcón LA, González-Cortés A, et al. Ischemia/reperfusion injury: pathophysiology, current clinical management, and potential preventive approaches. Mediators Inflamm. 2020;29(2020):8405370. https://doi.org/10.1155/2020/8405370
Cai J, Chen X, Liu X, et al. AMPK: the key to ischemia-reperfusion injury. J Cell Physiol. 2022;237(11):4079–96. https://doi.org/10.1002/jcp.30875
Ofir M, Arad M, Porat E, et al. Increased glycogen stores due to gamma-AMPK overexpression protects against ischemia and reperfusion damage. Biochem Pharmacol. 2008;75(7):1482–91. https://doi.org/10.1016/j.bcp.2007.12.011
Savchenko AS, Borissoff JI, Martinod K, et al. VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood. 2014;123(1):141–8. https://doi.org/10.1182/blood-2013-07-514992
Wallert M, Ziegler M, Wang X, et al. α-Tocopherol preserves cardiac function by reducing oxidative stress and inflammation in ischemia/reperfusion injury. Redox Biol. 2019;26:101292. https://doi.org/10.1016/j.redox.2019.101292
Li Y, Chen B, Yang X, et al. S100a8/a9 signaling causes mitochondrial dysfunction and cardiomyocyte death in response to ischemic/reperfusion injury. Circulation. 2019;140(9):751–64. https://doi.org/10.1161/CIRCULATIONAHA.118.039262
Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123(1):92–100. https://doi.org/10.1172/JCI62874
Ye J, Wang R, Wang M, et al. Hydroxysafflor yellow A ameliorates myocardial ischemia/reperfusion injury by suppressing calcium overload and apoptosis. Oxid Med Cell Longev. 2021;21(2021):6643615. https://doi.org/10.1155/2021/6643615
Yao H, Xie Q, He Q, et al. Pretreatment with panaxatriol saponin attenuates mitochondrial apoptosis and oxidative stress to facilitate treatment of myocardial ischemia-reperfusion injury via the regulation of Keap1/Nrf2 Activity. Oxid Med Cell Longev. 2022;2022:9626703. https://doi.org/10.1155/2022/9626703
Zhang H, Liu Y, Cao X, et al. Nrf2 promotes inflammation in early myocardial ischemia-reperfusion via recruitment and activation of macrophages. Front Immunol. 2021;12:763760. https://doi.org/10.3389/fimmu.2021.763760
Li L, Lin L, Lei S, et al. Maslinic acid inhibits myocardial ischemia-reperfusion injury-induced apoptosis and necroptosis via promoting autophagic flux. DNA Cell Biol. 2022;41(5):487–97. https://doi.org/10.1089/dna.2021.0918
Zhao WK, Zhou Y, Xu TT, et al. Ferroptosis: opportunities and challenges in myocardial ischemia-reperfusion injury. Oxid Med Cell Longev. 2021;23(2021):9929687. https://doi.org/10.1155/2021/9929687
Zhang Y, Liu D, Hu H, et al. HIF-1α/BNIP3 signaling pathway-induced-autophagy plays protective role during myocardial ischemia-reperfusion injury. Biomed Pharmacother. 2019;120:109464. https://doi.org/10.1016/j.biopha.2019.109464
Zhou WY, Cai ZR, Liu J, et al. Circular RNA: metabolism, functions and interactions with proteins. Mol Cancer. 2020;19(1):172. https://doi.org/10.1186/s12943-020-01286-3
Huang A, Zheng H, Wu Z, et al. Circular RNA-protein interactions: functions, mechanisms, and identification. Theranostics. 2020;10(8):3503–3517. https://doi.org/10.7150/thno.42174
Li J, Sun D, Pu W, et al. Circular RNAs in cancer: biogenesis, function, and clinical significance. Trends Cancer. 2020;6(4):319–36. https://doi.org/10.1016/j.trecan.2020.01.012
Gomes CPC, Schroen B, Kuster GM, et al. Regulatory RNAs in heart failure. Circulation. 2020;141(4):313–28. https://doi.org/10.1161/CIRCULATIONAHA.119.042474
Salzman J, Chen RE, Olsen MN, et al. Cell-type specific features of circular RNA expression [published correction appears in PLoS Genet. 2013;9(12). https://doi.org/10.1371/annotation/f782282b-eefa-4c8d-985c-b1484e845855
Chen LL. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol. 2020;21(8):475–90. https://doi.org/10.1038/s41580-020-0243-y
Li X, Yang L, Chen LL. The biogenesis, functions, and challenges of circular RNAs. Mol Cell. 2018;71(3):428-442. https://doi.org/10.1016/j.molcel.2018.06.034
Kristensen LS, Andersen MS, Stagsted LVW, et al. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20(11):675–91. https://doi.org/10.1038/s41576-019-0158-7
Bentley DL. Coupling mRNA processing with transcription in time and space. Nat Rev Genet. 2014;15(3):163–75. https://doi.org/10.1038/nrg3662
Zhang Y, Xue W, Li X, et al. The Biogenesis of Nascent Circular RNAs. Cell Rep. 2016;15(3):611–24. https://doi.org/10.1016/j.celrep.2016.03.058
Liang D, Tatomer DC, Luo Z, et al. The output of protein-coding genes shifts to circular RNAs when the pre-mRNA processing machinery is limiting. Mol Cell. 2017;68(5):940-954.e3. https://doi.org/10.1016/j.molcel.2017.10.034
Liang D, Wilusz JE. Short intronic repeat sequences facilitate circular RNA production. Genes Dev. 2014;28(20):2233–47. https://doi.org/10.1101/gad.251926.114
Jeck WR, Sorrentino JA, Wang K, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats [published correction appears in RNA. 2013 Mar;19(3):426]. RNA. 2013;19(2):141–157. https://doi.org/10.1261/rna.035667.112.
Zhang XO, Wang HB, Zhang Y, et al. Complementary sequence-mediated exon circularization. Cell. 2014;159(1):134–47. https://doi.org/10.1016/j.cell.2014.09.001
Guarnerio J, Bezzi M, Jeong JC, et al. Oncogenic Role of Fusion-circRNAs Derived from Cancer-Associated Chromosomal Translocations [published correction appears in Cell. 2016 Aug 11;166(4):1055–1056]. Cell. 2016;165(2):289–302. https://doi.org/10.1016/j.cell.2016.03.020
Ashwal-Fluss R, Meyer M, Pamudurti NR, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56(1):55–66. https://doi.org/10.1016/j.molcel.2014.08.019
Li X, Liu CX, Xue W, et al. Coordinated circRNA biogenesis and function with NF90/NF110 in viral infection. Mol Cell. 2017;67(2):214-227.e7. https://doi.org/10.1016/j.molcel.2017.05.023
Kramer MC, Liang D, Tatomer DC, et al. Combinatorial control of Drosophila circular RNA expression by intronic repeats, hnRNPs, and SR proteins. Genes Dev. 2015;29(20):2168–82. https://doi.org/10.1101/gad.270421.115
Conn SJ, Pillman KA, Toubia J, et al. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160(6):1125–1134. https://doi.org/10.1016/j.cell.2015.02.014
Starke S, Jost I, Rossbach O, et al. Exon circularization requires canonical splice signals. Cell Rep. 2015;10(1):103–11. https://doi.org/10.1016/j.celrep.2014.12.002
Altesha MA, Ni T, Khan A, Liu K, Zheng X. Circular RNA in cardiovascular disease. J Cell Physiol. 2019;234(5):5588–600. https://doi.org/10.1002/jcp.27384
Sanger HL, Klotz G, Riesner D, et al. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976;73(11):3852–6. https://doi.org/10.1073/pnas.73.11.3852
Liu J, Liu T, Wang X, et al. Circles reshaping the RNA world: from waste to treasure. Mol Cancer. 2017;16(1):58. https://doi.org/10.1186/s12943-017-0630-y
Salmena L, Poliseno L, Tay Y, et al. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language. Cell. 2011;146(3):353–8. https://doi.org/10.1016/j.cell.2011.07.014
Poliseno L, Salmena L, Zhang J, et al. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465(7301):1033–8. https://doi.org/10.1038/nature09144
Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–8. https://doi.org/10.1038/nature11993
Huang R, Zhang Y, Han B, et al. Circular RNA HIPK2 regulates astrocyte activation via cooperation of autophagy and ER stress by targeting MIR124–2HG [published correction appears in Autophagy. 2022 Jan;18(1):234] [published correction appears in Autophagy. 2020 Aug;16(8):1553] [published correction appears in Autophagy. 2020 Nov;16(11):2117–2118]. Autophagy. 2017;13(10):1722–1741. https://doi.org/10.1080/15548627.2017.1356975
Zheng Q, Bao C, Guo W, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:11215. https://doi.org/10.1038/ncomms11215.
Stoll L, Sobel J, Rodriguez-Trejo A, et al. Circular RNAs as novel regulators of β-cell functions in normal and disease conditions. Mol Metab. 2018;9:69–83. https://doi.org/10.1016/j.molmet.2018.01.010
Kristensen LS, Okholm TLH, Venø MT, et al. Circular RNAs are abundantly expressed and upregulated during human epidermal stem cell differentiation. RNA Biol. 2018;15(2):280–91. https://doi.org/10.1080/15476286.2017.1409931
Yu CY, Li TC, Wu YY, et al. The circular RNA circBIRC6 participates in the molecular circuitry controlling human pluripotency. Nat Commun. 2017;8(1):1149. https://doi.org/10.1038/s41467-017-01216-w.
Li Q, Pan X, Zhu D, et al. Circular RNA MAT2B promotes glycolysis and malignancy of hepatocellular carcinoma through the miR-338-3p/PKM2 axis under hypoxic stress. Hepatology. 2019;70(4):1298–316. https://doi.org/10.1002/hep.30671
Ma W, Wei D, Li X, et al. CircPCNX promotes PDGF-BB-induced proliferation and migration of human aortic vascular smooth muscle cells through regulating miR-1278/DNMT1 Axis. Cardiovasc Drugs Ther. 2023;37(5):877–89. https://doi.org/10.1007/s10557-022-07342-y
Liu J, Zhang X, Yu Z, et al. Circ_0026218 ameliorates oxidized low-density lipoprotein-induced vascular endothelial cell dysfunction by regulating miR-188–3p/TLR4/NF-κB pathway. Cardiovasc Drugs Ther. https://doi.org/10.1007/s10557-022-07416-x.
Li Z, Huang C, Bao C, et al. Exon-intron circular RNAs regulate transcription in the nucleus [published correction appears in Nat Struct Mol Biol. 2017;24(2):194]. Nat Struct Mol Biol. 2015;22(3):256–264. https://doi.org/10.1038/nsmb.2959
Du WW, Yang W, Chen Y, et al. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur Heart J. 2017;38(18):1402–12. https://doi.org/10.1093/eurheartj/ehw001
Zeng Y, Du WW, Wu Y, et al. A circular RNA binds to and activates AKT phosphorylation and nuclear localization reducing apoptosis and enhancing cardiac repair. Theranostics. 2017;7(16):3842–3855. https://doi.org/10.7150/thno.19764
Huang S, Li X, Zheng H, et al. Loss of super-enhancer-regulated circRNA Nfix induces cardiac regeneration after myocardial infarction in adult mice. Circulation. 2019;139(25):2857–76. https://doi.org/10.1161/CIRCULATIONAHA.118.038361
Chen CY, Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science. 1995;268(5209):415–7. https://doi.org/10.1126/science.7536344
Wang Y, Wang Z. Efficient backsplicing produces translatable circular mRNAs. RNA. 2015;21(2):172–9. https://doi.org/10.1261/rna.048272.114
Legnini I, Di Timoteo G, Rossi F, et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66(1):22–37.e9. https://doi.org/10.1016/j.molcel.2017.02.017
Pamudurti NR, Bartok O, Jens M, et al. Translation of CircRNAs. Mol Cell. 2017;66(1):9–21.e7. https://doi.org/10.1016/j. molcel.2017.02.021
Yang Y, Fan X, Mao M, et al. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 2017;27(5):626–41. https://doi.org/10.1038/cr.2017.31
Du WW, Fang L, Yang W, et al. Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ. 2017;24(2):357–70. https://doi.org/10.1038/cdd.2016.133.
Bertheloot D, Latz E, Franklin BS. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18(5):1106–21. https://doi.org/10.1038/s41423-020-00630-3
Badalzadeh R, Mokhtari B, Yavari R. Contribution of apoptosis in myocardial reperfusion injury and loss of cardioprotection in diabetes mellitus. J Physiol Sci. 2015;65(3):201–15. https://doi.org/10.1007/s12576-015-0365-8
Yang YN, Luo YB, Xu G, et al. CircHECTD1 promoted MIRI-associated inflammation via inhibiting miR-138-5p and upregulating ROCK2. Kaohsiung J Med Sci. 2023;39(7):675–87. https://doi.org/10.1002/kjm2.12686
Li X, Guo L, Wang J, et al. Pro-fibrotic and apoptotic activities of circARAP1 in myocardial ischemia-reperfusion injury. Eur J Med Res. 2023;28(1):84. https://doi.org/10.1186/s40001-023-01001-0
Liu X, Dou B, Tang W, et al. Cardioprotective effects of circ_0002612 in myocardial ischemia/reperfusion injury correlate with disruption of miR-30a-5p-dependent Ppargc1a inhibition. Int Immunopharmacol. 2023;117:110006. https://doi.org/10.1016/j.intimp.2023.110006
Yuan C, Lu J, Chen Z, et al. Circ-GTF2I/miR-590–5p axis aggravates myocardial ischemia-reperfusion injury by regulating Kelch repeat and BTB domain-containing protein 7. Evid Based Complement Alternat Med. 2022; 2022:2327669. https://doi.org/10.1155/2022/2327669
Li M, Ding W, Tariq MA, et al. A circular transcript of ncx1 gene mediates ischemic myocardial injury by targeting miR-133a-3p. Theranostics. 2018;8(21):5855–5869. https://doi.org/10.7150/thno.27285
Li D, You J, Mao C, et al. Circular RNA Fbxl5 regulates cardiomyocyte apoptosis during ischemia reperfusion injury via sponging microRNA-146a. J Inflamm Res. 2022;15:2539–2550. https://doi.org/10.2147/JIR.S360129
Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–41. https://doi.org/10.1016/j.cell.2011.10.026
Klionsky DJ, Petroni G, Amaravadi RK, et al. Autophagy in major human diseases. EMBO J. 2021;40(19):e108863. https://doi.org/10.15252/embj.2021108863
Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176(1–2):11–42. https://doi.org/10.1016/j.cell.2018.09.048
Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol. 2020;17(12):773–89. https://doi.org/10.1038/s41569-020-0403-y.
Popov SV, Mukhomedzyanov AV, Voronkov NS, et al. Regulation of autophagy of the heart in ischemia and reperfusion. Apoptosis. 2023;28(1–2):55–80. https://doi.org/10.1007/s10495-022-01786-1
Zhang CL, Long TY, Bi SS, et al. CircPAN3 ameliorates myocardial ischaemia/reperfusion injury by targeting miR-421/Pink1 axis-mediated autophagy suppression [published correction appears in Lab Invest. 2021 Jan 22;]. Lab Invest. 2021;101(1):89–103. https://doi.org/10.1038/s41374-020-00483-4
Huang C, Shu L, Zhang H, et al. Circ_ZNF512-mediated miR-181d-5p inhibition limits cardiomyocyte autophagy and promotes myocardial ischemia/reperfusion injury through an EGR1/mTORC1/TFEB-based mechanism. J Med Chem. 2022;65(3):1808–21. https://doi.org/10.1021/acs.jmedchem.1c00745
Jin P, Li LH, Shi Y, et al. Salidroside inhibits apoptosis and autophagy of cardiomyocyte by regulation of circular RNA hsa_circ_0000064 in cardiac ischemia-reperfusion injury. Gene. 2021;767:145075. https://doi.org/10.1016/j.gene.2020.145075
Sun G, Shen JF, Wei XF, et al. Circular RNA Foxo3 relieves myocardial ischemia/reperfusion injury by suppressing autophagy via inhibiting HMGB1 by repressing KAT7 in myocardial infarction. J Inflamm Res. 2021;14:6397–6407. https://doi.org/10.2147/JIR.S339133
Zhou LY, Zhai M, Huang Y, et al. The circular RNA ACR attenuates myocardial ischemia/reperfusion injury by suppressing autophagy via modulation of the Pink1/ FAM65B pathway. Cell Death Differ. 2019;26(7):1299–315. https://doi.org/10.1038/s41418-018-0206-4
Qiu Z, Wang Y, Liu W, et al. CircHIPK3 regulates the autophagy and apoptosis of hypoxia/reoxygenation-stimulated cardiomyocytes via the miR-20b-5p/ATG7 axis. Cell Death Discov. 2021;7(1):64. https://doi.org/10.1038/s41420-021-00448-6
Bagheri F, Khori V, Alizadeh AM, et al. Reactive oxygen species-mediated cardiac-reperfusion injury: mechanisms and therapies. Life Sci. 2016;165:43–55. https://doi.org/10.1016/j.lfs.2016.09.013
Mokhtari-Zaer A, Marefati N, Atkin SL, et al. The protective role of curcumin in myocardial ischemia-reperfusion injury. J Cell Physiol. 2018;234(1):214–22. https://doi.org/10.1002/jcp.26848
Zheng H, Huang S, Wei G, et al. CircRNA Samd4 induces cardiac repair after myocardial infarction by blocking mitochondria-derived ROS output. Mol Ther. 2022;30(11):3477–98. https://doi.org/10.1016/j.ymthe.2022.06.016
Wang L, Wang C, Sun Z, et al. Knockdown of Mmu-circ-0001380 attenuates myocardial ischemia/reperfusion injury via modulating miR-106b-5p/Phlpp2 axis. J Cardiovasc Transl Res. 2023;16(5):1064–77. https://doi.org/10.1007/s12265-023-10383-9
Jin L, Zhang Y, Jiang Y, et al. Circular RNA Rbms1 inhibited the development of myocardial ischemia reperfusion injury by regulating miR-92a/BCL2L11 signaling pathway. Bioengineered. 2022;13(2):3082–92. https://doi.org/10.1080/21655979.2022.2025696
Liu J, Dong W, Gao C, et al. Salvianolic acid B protects cardiomyocytes from ischemia/reperfusion injury by mediating circTRRAP/miR-214-3p/SOX6 axis. Int Heart J. 2022;63(6):1176–86. https://doi.org/10.1536/ihj.22-102
Wang L, Su H, Liu W. Hsa_circ_0010729 regulates H2O2-induced myocardial injury by regulating miR-1184/RIPK1 axis. Transpl Immunol. 2022; 74:101653. https://doi.org/10.1016/j.trim.2022.101653.
Biswas SK. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016;2016:5698931. https://doi.org/10.1155/2016/5698931
Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339(6116):161–6. https://doi.org/10.1126/science.1230719
Kusuoka H, Porterfield JK, Weisman HF, et al. Pathophysiology and pathogenesis of stunned myocardium. Depressed Ca2+ activation of contraction as a consequence of reperfusion-induced cellular calcium overload in ferret hearts. J Clin Invest. 1987;79(3):950–961. https://doi.org/10.1172/JCI112906
Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015;6:524–51. https://doi.org/10.1016/j.redox.2015.08.020
Sandanger Ø, Ranheim T, Vinge LE, et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2013;99(1):164–74. https://doi.org/10.1093/cvr/cvt091
Martínez GJ, Celermajer DS, Patel S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation [published correction appears in Atherosclerosis. 2018; 273:157]. Atherosclerosis. 2018; 269:262-271https://doi.org/10.1016/j.atherosclerosis.2017.12.027
Algoet M, Janssens S, Himmelreich U, et al. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc Med. 2023;33(6):357–366. https://doi.org/10.1016/j.tcm.2022.02.005
Jin A, Zhang Q, Cheng H, et al. Circ_0050908 up-regulates TRAF3 by sponging miR-324–5p to aggravate myocardial ischemia-reperfusion injury. Int Immunopharmacol. 2022;108:108740. https://doi.org/10.1016/j.intimp.2022.108740
Hu X, Ma R, Cao J, et al. CircSAMD4A aggravates H/R-induced cardiomyocyte apoptosis and inflammatory response by sponging miR-138-5p. J Cell Mol Med. 2022;26(6):1776–84. https://doi.org/10.1111/jcmm.16093
Zhu Y, Zou C, Jia Y, et al. Knockdown of circular RNA circMAT2B reduces oxygen-glucose deprivation-induced inflammatory injury in H9c2 cells through up-regulating miR-133. Cell Cycle. 2020;19(20):2622–30. https://doi.org/10.1080/15384101.2020.1814025
Zhang C, Zhang B. RNA therapeutics: updates and future potential. Sci China Life Sci. 2023;66(1):12–30. https://doi.org/10.1007/s11427-022-2171-2
Brenner S, Jacob F, Meslson M. An unstable intermediate carrying information from genes to ribosomes for protein synthesis. Nature. 1961;190:576–81. https://doi.org/10.1038/190576a0
Chow LT, Gelinas RE, Broker TR, et al. An amazing sequence arrangement at the 5’ ends of adenovirus 2 messenger RNA. Cell. 1977;12(1):1–8. https://doi.org/10.1016/0092-8674(77)90180-5
Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. https://doi.org/10.1016/0092-8674(93)90529-y
Jinek M, Chylinski K, Fonfara I, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–21. https://doi.org/10.1126/science.1225829
Anthony K. RNA-based therapeutics for neurological diseases. RNA Biol. 2022;19(1):176–90. https://doi.org/10.1080/15476286.2021.2021650
Kristensen LS, Jakobsen T, Hager H, et al. The emerging roles of circRNAs in cancer and oncology. Nat Rev Clin Oncol. 2022;19(3):188–206. https://doi.org/10.1038/s41571-021-00585-y
Bougea A, Stefanis L. microRNA and circRNA in Parkinson’s disease and atypical parkinsonian syndromes. Adv Clin Chem. 2023;115:83–133. https://doi.org/10.1016/bs.acc.2023.03.002
Zhao Q, Liu J, Deng H, et al. Targeting Mitochondria-Located circRNA SCAR Alleviates NASH via Reducing mROS Output. Cell. 2020;183(1):76-93.e22. https://doi.org/10.1016/j.cell.2020.08.009
Fan W, Pang H, Xie Z, et al. Circular RNAs in diabetes mellitus and its complications. Front Endocrinol (Lausanne). 2022;13:885650. https://doi.org/10.3389/fendo.2022.885650
Wang K, Gao XQ, Wang T, et al. The Function and Therapeutic Potential of Circular RNA in Cardiovascular Diseases. Cardiovasc Drugs Ther. 2023;37(1):181–98. https://doi.org/10.1007/s10557-021-07228-5
Liu X, Zhang Y, Zhou S, et al. Circular RNA: An emerging frontier in RNA therapeutic targets, RNA therapeutics, and mRNA vaccines. J Control Release. 2022;348:84–94. https://doi.org/10.1016/j.jconrel.2022.05.043
Lavenniah A, Luu TDA, Li YP, et al. Engineered circular RNA sponges act as miRNA inhibitors to attenuate pressure overload-induced cardiac hypertrophy. Mol Ther. 2020;28(6):1506–17. https://doi.org/10.1016/j.ymthe.2020.04.006
Chang H, Li ZB, Wu JY, et al. Circ-100338 induces angiogenesis after myocardial ischemia-reperfusion injury by sponging miR-200a-3p. Eur Rev Med Pharmacol Sci. 2020;24(11):6323–6332. https://doi.org/10.26355/eurrev_202006_21530.
Huang C, Qu Y, Feng F, et al. Cardioprotective effect of circ_SMG6 knockdown against myocardial ischemia/reperfusion injury correlates with miR-138–5p-Mediated EGR1/TLR4/TRIF inactivation. Oxid Med Cell Longev. 2022;2022:1927260. https://doi.org/10.1155/2022/1927260
Xiao Y, Oumarou DB, Wang S, et al. Circular RNA involved in the protective effect of Malva sylvestris L. on myocardial ischemic/re-perfused injury. Front Pharmacol. 2020;11:520486. https://doi.org/10.3389/fphar.2020.520486
Abe N, Matsumoto K, Nishihara M, et al. Rolling Circle Translation of Circular RNA in Living Human Cells. Sci Rep. 2015;5:16435. https://doi.org/10.1038/srep16435
Yu L, Liu Y. circRNA_0016624 could sponge miR-98 to regulate BMP2 expression in postmenopausal osteoporosis. Biochem Biophys Res Commun. 2019;516(2):546–550. https://doi.org/10.1016/j.bbrc.2019.06.087
Loan Young T, Chang Wang K, James Varley A, et al. Clinical delivery of circular RNA: lessons learned from RNA drug development. Adv Drug Deliv Rev. 2023;197:114826. https://doi.org/10.1016/j.addr.2023.114826
Wesselhoeft RA, Kowalski PS, Anderson DG. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat Commun. 2018;9(1):2629. https://doi.org/10.1038/s41467-018-05096-6.
Liu GW, Guzman EB, Menon N, et al. Lipid nanoparticles for nucleic acid delivery to endothelial cells. Pharm Res. 2023;40(1):3–25. https://doi.org/10.1007/s11095-023-03471-7
Liu CX, Chen LL. Circular RNAs: Characterization, cellular roles, and applications [published correction appears in Cell. 2022 Jun 23;185(13):2390]. Cell. 2022;185(12):2016–2034. https://doi.org/10.1016/j.cell.2022.04.021
Funding
This work was supported by grant from the National Natural Science foundation of China (No.82170286; No.81960051); Construction Funding from Characteristic Key Laboratory of Guizhou Province, No. [2021]313.
Author information
Authors and Affiliations
Contributions
The conceptualization of this review was spearheaded by Kaiyuan Chen, while Junhou Lu and Guiyou Liang conducted an extensive literature search. Kaiyuan Chen took the lead in drafting the manuscript, with critical editing contributions from Zhou Liu and Guiyou Liang. Hu Xuanyi and Yang Siyuan are responsible for language proofreading. All authors diligently reviewed and provided their approval for the final version of the manuscript.
Corresponding author
Ethics declarations
Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Ethic Approval
Not applicable.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent to Participate
Not applicable.
Consent to Publish
The authors have reviewed the final version of the manuscript and approve it for publication. To the best of our knowledge and belief, this manuscript has not been published in whole or in part nor is it being considered for publication elsewhere.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, KY., Liu, Z., Lu, JH. et al. The Function of Circular RNAs in Myocardial Ischemia–Reperfusion Injury: Underlying Mechanisms and Therapeutic Advancement. Cardiovasc Drugs Ther (2024). https://doi.org/10.1007/s10557-024-07557-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-024-07557-1