Abstract
Background
In the Asian population, the presence of the CYP2C19 loss-of-function (LOF) allele is a known genetic variation. This allele is associated with a reduced capacity to metabolize clopidogrel into its active forms through the CYP2C19 enzyme, resulting in diminished platelet inhibition and an elevated risk of recurrent cardiovascular events. Regulatory authorities have recommended an alternative P2Y12 inhibitor, ticagrelor, for individuals carrying the LOF allele. Consequently, this study seeks to assess the impact of the CYP2C19 genotype on the Platelet reactivity index (PRI) using a rapid genetic testing approach in Asian patients with chronic coronary syndromes (CCS) who undergo percutaneous coronary intervention (PCI).
Methods
This prospective study employed a parallel design, single-center design, and randomized approach. Genotyping for the CYP2C19*2 and *3 polymorphisms was conducted using the Nested Allele-Specific Multiplex PCR (NASM-PCR) technique. Patients meeting the inclusion criteria underwent genotyping for CYP2C19 polymorphisms. Following PCI, patients were randomly assigned to receive either ticagrelor or clopidogrel. PRI assessments were performed four hours after loading dose administration. The trial was registered with ClinicalTrials.gov under the identifier NCT05516784.
Results
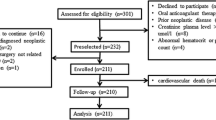
Among the 94 patients recruited for the study, 40 (42.55%) were identified as carriers of the LOF allele for CYP2C19*2 and *3 (*1/*2, *2/*2, *1/*3). Out of the 84 patients evaluated for PRI (44 receiving clopidogrel and 40 receiving ticagrelor), 21 (47.7%) of the clopidogrel group and 39 (97.5%) of the ticagrelor group exhibited a favorable response to antiplatelet therapy (PRI < 50). Patients treated with ticagrelor demonstrated superior antiplatelet responses compared to those receiving clopidogrel, regardless of LOF carrier status (P = 0.005 and < 0.001 for non-LOF and LOF carriers, respectively).
Conclusion
NASM-PCR as a rapid genetic test holds promise for personalizing antiplatelet therapy in Asian CCS patients.


Similar content being viewed by others
Data Availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Members WC et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines Journal of the American College of Cardiology, 2022. 79(2): p. e21-e129.
Valgimigli M, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-thoracic surgery (EACTS). Eur Heart J. 2018;39(3):213–60.
Lee S-Y, et al. Short-term versus long-term dual antiplatelet therapy after drug-eluting stent implantation in elderly patients: a meta-analysis of individual participant data from 6 randomized trials. Cardiovasc Interventions. 2018;11(5):435–43.
Akkaif MA, et al. The role of genetic polymorphism and other factors on Clopidogrel Resistance (CR) in an Asian Population with Coronary Heart Disease (CHD). Molecules. 2021;26(7):1987.
Amin AM, et al. The effect of CYP2C19 genetic polymorphism and non-genetic factors on clopidogrel platelets inhibition in east Asian coronary artery disease patients. Thromb Res. 2017;158:22–4.
Tamargo J, et al. Racial and ethnic differences in pharmacotherapy to prevent coronary artery disease and thrombotic events. Eur Heart Journal-Cardiovascular Pharmacotherapy. 2022;8(7):738–51.
Wallentin L, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57.
Jourdi G, et al. Antiplatelet Therapy for Atherothrombotic Disease in 2022—From Population to patient-centered approaches. Front Cardiovasc Med. 2022;9:805525.
Dhillon S. Ticagrelor: a review of its use in adults with acute coronary syndromes. Am J Cardiovasc Drugs. 2015;15(1):51–68.
Yoon HY, et al. Efficacy and safety of clopidogrel versus prasugrel and ticagrelor for coronary artery disease treatment in patients with CYP2C19 LoF alleles: a systemic review and meta-analysis. Br J Clin Pharmacol. 2020;86(8):1489–98.
Biswas M, et al. Risk of major adverse cardiovascular events of CYP2C19 loss-of-function genotype guided prasugrel/ticagrelor vs clopidogrel therapy for acute coronary syndrome patients undergoing percutaneous coronary intervention: a meta-analysis. Platelets. 2021;32(5):591–600.
Akkaif MA, et al. A review of the effects of ticagrelor on adenosine concentration and its clinical significance. Pharmacol Rep. 2021;73(6):1551–64.
Aradi D et al. Platelet reactivity and clinical outcomes in acute coronary syndrome patients treated with prasugrel and clopidogrel: a pre-specified exploratory analysis from the TROPICAL-ACS trial European heart journal, 2019. 40(24): p. 1942–51.
Li Q, et al. IMPACT OF RENAL DYSFUNCTION ON RESIDUAL PLATELET REACTIVITY AND CLINICAL OUTCOMES IN PATIENTS WITH ACUTE CORONARY SYNDROME TREATED WITH CLOPIDOGREL. J Am Coll Cardiol. 2021;77(18Supplement1):198–8.
Li P, et al. Ticagrelor overcomes high platelet reactivity in patients with acute myocardial infarction or coronary artery in-stent restenosis: a randomized controlled trial. Sci Rep. 2015;5(1):1–8.
Chen D-Y, et al. Paraoxonase-1 is not a major determinant of stent thrombosis in a Taiwanese population. PLoS ONE. 2012;7(6):e39178.
Duarte JD, Cavallari LH. Pharmacogenetics to guide cardiovascular drug therapy. Nat Reviews Cardiol. 2021;18(9):649–65.
Lee CR, et al. Clinical pharmacogenetics implementation Consortium guideline for CYP2C19 genotype and clopidogrel therapy: 2022 update. Volume 112. Clinical Pharmacology & Therapeutics; 2022. pp. 959–67. 5.
Wang Z, et al. Two common mutations within CYP2C19 affected platelet aggregation in Chinese patients undergoing PCI: a one-year follow-up study. Pharmacogenomics J. 2019;19(2):157–63.
Ahmed S, et al. Antiplatelet response to clopidogrel is associated with a haplotype in CYP2C19 gene in Pakistani patients. Sci Rep. 2022;12(1):1–10.
Beitelshees AL, et al. CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention in diverse clinical settings. J Am Heart Association. 2022;11(4):e024159.
Yu L, et al. Association between cytochrome P450 2C19 polymorphism and clinical outcomes in clopidogrel-treated Uygur population with acute coronary syndrome: a retrospective study. BMC Cardiovasc Disord. 2021;21(1):1–10.
Zhang Y, et al. Clinical outcomes after percutaneous coronary intervention over time on the basis of CYP2C19 polymorphisms. J Cardiovasc Pharmacol. 2022;79(2):183–91.
Yamani N, et al. Does individualized guided selection of antiplatelet therapy improve outcomes after percutaneous coronary intervention? A systematic review and meta-analysis. Annals of Medicine and Surgery; 2022. p. 103964.
Mega JL, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304(16):1821–30.
Shuldiner AR, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302(8):849–57.
Sorich MJ, et al. CYP2C19 genotype has a greater effect on adverse cardiovascular outcomes following percutaneous coronary intervention and in Asian populations treated with clopidogrel: a meta-analysis. Circulation: Cardiovasc Genet. 2014;7(6):895–902.
Jang J-S, et al. Meta-analysis of cytochrome P450 2C19 polymorphism and risk of adverse clinical outcomes among coronary artery disease patients of different ethnic groups treated with clopidogrel. Am J Cardiol. 2012;110(4):502–8.
Zhang L, et al. Genetic determinants of high on-treatment platelet reactivity in clopidogrel treated Chinese patients. Thromb Res. 2013;132(1):81–7.
Tang X-F, et al. Effect of the CYP2C19* 2 and* 3 genotypes, ABCB1 C3435T and PON1 Q192R alleles on the pharmacodynamics and adverse clinical events of clopidogrel in Chinese people after percutaneous coronary intervention. Eur J Clin Pharmacol. 2013;69:1103–12.
Li-Wan‐Po A, et al. Pharmacogenetics of CYP2C19: functional and clinical implications of a new variant CYP2C19* 17. Br J Clin Pharmacol. 2010;69(3):222–30.
Ieiri I, et al. Pharmacokinetics of omeprazole (a substrate of CYP2C19) and comparison with two mutant alleles, CYP2C19m1 in exon 5 and CYP2C19m2 in exon 4, in Japanese subjects. Volume 59. Clinical Pharmacology & Therapeutics; 1996. pp. 647–53. 6.
Yin OQ, et al. Omeprazole as a CYP2C19 marker in Chinese subjects: assessment of its gene-dose effect and intrasubject variability. J Clin Pharmacol. 2004;44(6):582–9.
Food, Administration D. Drug safety communication: Reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drughttp://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203888. htm, 2010.
Use C. f.M.P.f.H., European Medicines Agency. Summary of product characteristics ribociclib. 2017. 2020.
Moon JY, et al. Role of genetic testing in patients undergoing percutaneous coronary intervention. Expert Rev Clin Pharmacol. 2018;11(2):151–64.
Empey PE, et al. Multisite investigation of strategies for the implementation of CYP2C19 genotype-guided antiplatelet therapy. Clin Pharmacol Ther. 2018;104(4):664–74.
Pereira N, et al. Clopidogrel Pharmacogenetics Circ Cardiovasc Interv. 2019;12(4):e007811.
Adamski P, et al. Diurnal variability of platelet aggregation in patients with myocardial infarction treated with Prasugrel and Ticagrelor. J Clin Med. 2022;11(4):1124.
Holmes DR, et al. ACCF/AHA clopidogrel clinical alert: approaches to the FDA boxed warning a report of the American college of cardiology foundation task force on clinical expert consensus documents and the American heart association endorsed by the society for cardiovascular angiography and interventions and the society of thoracic surgeons. J Am Coll Cardiol. 2010;56(4):321–41.
Silvain J, et al. Ticagrelor versus clopidogrel in elective percutaneous coronary intervention (ALPHEUS): a randomised, open-label, phase 3b trial. The Lancet. 2020;396(10264):1737–44.
Aradi D, et al. Bleeding and stent thrombosis on P2Y12-inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J. 2015;36(27):1762–71.
Cavallari LH, et al. Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. JACC: Cardiovasc Interventions. 2018;11(2):181–91.
Akkaif MA, et al. Investigate the strategy of using pharmacogenetics and pharmacometabonomics to the personalization of ticagrelor antiplatelet therapy. Syst Reviews Pharm. 2020;11(9):1100–7.
Desta Z, et al. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet. 2002;41(12):913–58.
Hwang S-J, et al. The cytochrome 2C19* 2 and* 3 alleles attenuate response to clopidogrel similarly in east Asian patients undergoing elective percutaneous coronary intervention. Thromb Res. 2011;127(1):23–8.
Qian W, et al. Comparison of ticagrelor and clopidogrel in the treatment of patients with coronary heart disease carrying CYP2C19 loss of function allele. J Thorac Disease. 2022;14(7):2591.
Gandhi A. Horenstein RB. Association of Cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–58. Ryan K.
Xu F, et al. Antiplatelet effects of ticagrelor versus clopidogrel after coronary artery bypass graft surgery: a single-center randomized controlled trial. J Thorac Cardiovasc Surg. 2019;158(2):430–7. e4.
Franchi F, et al. Prasugrel versus Ticagrelor in patients with CYP2C19 loss-of-function genotypes: results of a randomized pharmacodynamic study in a feasibility investigation of rapid genetic testing. Basic to Translational Science. 2020;5(5):419–28.
Su J et al. ABCB1 C3435T polymorphism and response to clopidogrel treatment in coronary artery disease (CAD) patients: a meta-analysis 2012.
Tan SSN, et al. Association of CYP2C19* 2 polymorphism with clopidogrel response and 1-year major adverse cardiovascular events in a multiethnic population with drug-eluting stents. Pharmacogenomics. 2017;18(13):1225–39.
Sibbing D, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC: Cardiovasc Interventions. 2019;12(16):1521–37.
Castrichini M, Luzum JA, Pereira N. Pharmacogenetics of Antiplatelet Therapy. Annual Review of Pharmacology and Toxicology; 2022. p. 63.
Zhu W-Y, et al. Association of CYP2C19 polymorphisms with the clinical efficacy of clopidogrel therapy in patients undergoing carotid artery stenting in Asia. Sci Rep. 2016;6(1):1–7.
Bergmeijer TO, et al. Feasibility and implementation of CYP2C19 genotyping in patients using antiplatelet therapy. Pharmacogenomics. 2018;19(7):621–8.
Claassens DM, et al. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. N Engl J Med. 2019;381(17):1621–31.
Brandt JT, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5(12):2429–36.
Sofi F, et al. Cytochrome P450 2C19* 2 polymorphism and cardiovascular recurrences in patients taking clopidogrel: a meta-analysis. Pharmacogenomics J. 2011;11(3):199–206.
ten Berg JM, Deneer VH. Does CYP2C19 genotype affect clinical outcome? Nat Reviews Cardiol. 2012;9(4):192–4.
Galli M, et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. The Lancet. 2021;397(10283):1470–83.
Gower MN, et al. Clinical utility of CYP2C19 genotype-guided antiplatelet therapy in patients at risk of adverse cardiovascular and cerebrovascular events: a review of emerging evidence. Pharmacogenomics and Personalized Medicine. 2020;13:239.
Notarangelo FM, et al. Pharmacogenomic approach to selecting antiplatelet therapy in patients with acute coronary syndromes: the PHARMCLO trial. J Am Coll Cardiol. 2018;71(17):1869–77.
Pereira NL, et al. Effect of CYP2C19 genotype on ischemic outcomes during oral P2Y12 inhibitor therapy: a meta-analysis. Cardiovasc Interventions. 2021;14(7):739–50.
Shen D-L, et al. Clinical value of CYP2C19 genetic testing for guiding the antiplatelet therapy in a Chinese population. J Cardiovasc Pharmacol. 2016;67(3):232–6.
Pereira NL, et al. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: the TAILOR-PCI randomized clinical trial. JAMA. 2020;324(8):761–71.
Janssen PW, et al. Tailored P2Y12 inhibitor treatment in patients undergoing non-urgent PCI—The POPular risk score study. Eur J Clin Pharmacol. 2019;75(9):1201–10.
Sánchez-Ramos J, et al. Results of genotype-guided antiplatelet therapy in patients who undergone percutaneous coronary intervention with stent. Int J Cardiol. 2016;225:289–95.
Angiolillo DJ, et al. Use of the VerifyNow point of care assay to assess the pharmacodynamic effects of loading and maintenance dose regimens of prasugrel and ticagrelor. J Thromb Thrombolysis. 2021;51(3):741–7.
Mehta RH, et al. Doing the right things and doing them the right way: association between hospital guideline adherence, dosing safety, and outcomes among patients with acute coronary syndrome. Circulation. 2015;131(11):980–7.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
M.A.A., N.A.A.D., and B.I.: Conceptualization; M.A.A., N.A.A.D., D.A.M.N., M.A.S, and B.I.: Methodology; M.A.A. did the analysis, and wrote the original draft as well as the final manuscript; N.A.A.D., A.S., D.A.M.N., M.A.S, M.J.A.W., and B.I.: critically reviewed the manuscript; N.A.A.D., and B.I.: Supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
The study protocol was submitted to the National Institute of Health (NIH), Ministry of Health of Malaysia, and had been reviewed and granted ethical approval by the Medical Research and Ethics Committee (MREC) (NMRR-20-2417-56795). All patients provided written informed consent for participation before study entry.
Consent for Publication
All authors reviewed the manuscript and approved its submission.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akkaif, M.A., Daud, N.A.A., Noor, D.A.M. et al. The Impact of CYP2C19 Genotype on the Platelet Reactivity Index (PRI) among Chronic Coronary Syndromes (CCS) Patients Undergoing Percutaneous Coronary Intervention (PCI): Affectability of Rapid Genetic Testing. Cardiovasc Drugs Ther (2024). https://doi.org/10.1007/s10557-024-07544-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-024-07544-6