Abstract
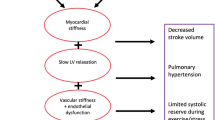
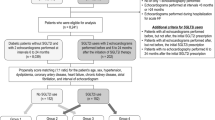
Heart failure with preserved ejection fraction (HFpEF) is now the most common form of heart failure (HF). This syndrome is associated with an elevated morbi-mortality, and effective therapies are urgently needed. Sodium-glucose co-transporter 2 inhibitors (SGLT2i) are the first pharmacological class that has demonstrated to reduce hospitalization and cardiovascular mortality in large clinical trials in HFpEF. Furthermore, the dual SGLT 1/2 inhibitor sotagliflozin has shown a reduction in cardiovascular outcomes in diabetic HF patients, regardless of ejection fraction Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) Trial, and prevents the development of HF in patients with diabetes and chronic kidney disease Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk (SCORED) trial. The major objective of the Sotagliflozin in Heart Failure With Preserved Ejection Fraction Patients (SOTA-P-CARDIA) trial (NCT05562063) is to investigate whether the observed cardiorenal benefits of sotagliflozin in HF patients with diabetes can be extended to a non-diabetic population. The SOTA-P-CARDIA is a prospective, randomized, double-blinded, placebo-controlled study that will randomize non-diabetic patients with the universal definition of HFpEF (ejection fraction > 50% assessed the day of randomization). Qualifying patients will be randomized, in blocks of 4, to receive either sotagliflozin or placebo for a period of 6 months. The primary outcome is changes in left ventricular mass by cardiac magnetic resonance from randomization to end of the study between the groups. Secondary end points include changes in peak VO2; myocardial mechanics, interstitial myocardial fibrosis, and volume of epicardial adipose tissue; distance in the 6-min walk test; and quality of life. Finally, the authors expect that this trial will help to clarify the potential benefits of the use of sotagliflozin in non-diabetic HFpEF patients.


Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
van Riet EES, Hoes AW, Wagenaar KP, Limburg A, Landman MAJ, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time A systematic review. Eur J Heart Fail. 2016;18:242–52.
McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726.
Heidenreich PA, Bozkurt B, Aguilar D, et al. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e876–94.
Simmonds SJ, Cuijpers I, Heymans S, Jones EAV. Cellular and molecular differences between HFpEF and HFrEF: a step ahead in an improved pathological understanding. Cells. 2020;9:242.
Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71.
Pistelli L, Parisi F, Correale M, et al. Gliflozins: from antidiabetic drugs to cornerstone in heart failure therapy-a boost to their utilization and multidisciplinary approach in the management of heart failure. J Clin Med. 2023;12:379.
Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145:e153-639.
Packer M, Zannad F, Anker SD. Heart failure and a preserved ejection fraction: a side-by-side examination of the PARAGON-HF and EMPEROR-Preserved trials. Circulation. 2021;144:1193–5.
Pitt B, Bhatt DL, Metra M. Does SGLT1 inhibition add to the benefits of SGLT2 inhibition in the prevention and treatment of heart failure? Eur Heart J. 2022;43:4754–7.
Cinti F, Moffa S, Impronta F, et al. Spotlight on ertugliflozin and its potential in the treatment of type 2 diabetes: evidence to date. Drug Des Devel Ther. 2017;11:2905–19.
Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–61.
Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–98.
Savarese G, Stolfo D, Sinagra G, Lund LH. Heart failure with mid-range or mildly reduced ejection fraction. Nat Rev Cardiol. 2022;19:100–16.
Lam CSP, Voors AA, Piotr P, McMurray JJV, Solomon SD. Time to rename the middle child of heart failure: heart failure with mildly reduced ejection fraction. Eur Heart J. 2020;41:2353–5.
Packer M. Role of deranged energy deprivation signaling in the pathogenesis of cardiac and renal disease in states of perceived nutrient overabundance. Circulation. 2020;141:2095–105.
Packer M. SGLT2 inhibitors: role in protective reprogramming of cardiac nutrient transport and metabolism. Nat Rev Cardiol. 2023. https://doi.org/10.1038/s41569-022-00824-4.
Santos-Gallego CG, Vahl TP, Goliasch G, et al. Sphingosine-1-phosphate receptor agonist fingolimod increases myocardial salvage and decreases adverse postinfarction left ventricular remodeling in a porcine model of ischemia/reperfusion. Circulation. 2016;133:954–66.
Ishikawa K, Aguero J, Tilemann L, et al. Characterizing preclinical models of ischemic heart failure: differences between LAD and LCx infarctions. Am J Physiol Heart Circ Physiol. 2014;307(10):H1478–86.
Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73(15):1931–44.
Santos-Gallego CG, Mayr M, Badimon J. SGLT2 inhibitors in heart failure: targeted metabolomics and energetic metabolism. Circulation. 2022;146(11):819–21.
Burrage MK, Hundertmark M, Valkovič L, et al. Energetic basis for exercise-induced pulmonary congestion in heart failure with preserved ejection fraction. Circulation. 2021;144(21):1664–78.
Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, et al. Empagliflozin ameliorates diastolic dysfunction and left ventricular fibrosis/stiffness in nondiabetic heart failure: a multimodality study. JACC Cardiovasc Imaging. 2021;14(2):393–407.
Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77:243–55.
Requena-Ibáñez JA, Santos-Gallego CG, Rodriguez-Cordero A, et al. Empagliflozin improves quality of life in nondiabetic HFrEF patients Sub-analysis of the EMPATROPISM trial. Diabetes Metab Syndr. 2022;16(2):102417.
Requena-Ibáñez JA, Santos-Gallego CG, Rodriguez-Cordero A, et al. Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: from the EMPA-TROPISM Study. JACC Heart Fail. 2021;9:578–89.
Pugliese NR, Paneni F, Mazzola M, et al. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail. 2021;23:1858–71.
Tromp J, Packer M, Lam CS. The diverging role of epicardial adipose tissue in heart failure with reduced and preserved ejection fraction: not all fat is created equal. Eur J Heart Fail. 2021;23:1872–4.
Gorter TM, van Woerden G, Rienstra M, et al. Epicardial adipose tissue and invasive hemodynamics in heart failure with preserved ejection fraction. JACC Heart Fail. 2020;8:667–76.
Koepp KE, Obokata M, Reddy YNV, Olson TP, Borlaug BA. Hemodynamic and functional impact of epicardial adipose tissue in heart failure with preserved ejection fraction. JACC Heart Fail. 2020;8:657–66.
Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71:2360–72.
Packer M. Differential pathophysiological mechanisms in heart failure with a reduced or preserved ejection fraction in diabetes. JACC Heart Fail. 2021;9:535–49.
Di Franco A, Cantini G, Tani A, et al. Sodium-dependent glucose transporters (SGLT) in human ischemic heart: a new potential pharmacological target. Int J Cardiol. 2017;243:86–90.
Sayour AA, Ruppert M, Oláh A, et al. Effects of SGLT2 inhibitors beyond glycemic control-focus on myocardial SGLT1. Int J Mol Sci. 2021;22:9852.
Seidelmann SB, Feofanova E, Yu B, et al. Genetic variants in SGLT1, glucose tolerance, and cardiometabolic risk. J Am Coll Cardiol. 2018;72:1763–73.
Lin H, Guan L, Meng L, Uzui H, Guo H. SGLT1 knockdown attenuates cardiac fibroblast activation in diabetic cardiac fibrosis. Front Pharmacol. 2021;12:700366.
Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–28.
Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384:129–39.
Zheng C, Lin M, Chen Y, Xu H, Yan L, Dai H. Effects of sodium-glucose cotransporter type 2 inhibitors on cardiovascular, renal, and safety outcomes in patients with cardiovascular disease: a meta-analysis of randomized controlled trials. Cardiovasc Diabetol. 2021;20:83.
Gilbert RE, Connelly KA. Reduction in the incidence of myocardial infarction with sodium-glucose linked cotransporter-2 inhibitors: evident and plausible. Cardiovasc Diabetol. 2019;18:6.
McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148–58.
Li Y, Xu G. Sodium glucose cotransporter 1 (SGLT1) inhibitors in cardiovascular protection: Mechanism progresses and challenges. Pharmacol Res. 2022;176:106049.
Cefalo CMA, Cinti F, Moffa S, et al. Sotagliflozin, the first dual SGLT inhibitor: current outlook and perspectives. Cardiovasc Diabetol. 2019;18:20.
Rekhraj S, Gandy SJ, Szwejkowski BR, et al. High-dose allopurinol reduces left ventricular mass in patients with ischemic heart disease. J Am Coll Cardiol. 2013;61:926–32.
Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975.
Andreini D, Conte E, Mushtaq S, et al. Comprehensive evaluation of left ventricle dysfunction by a new computed tomography scanner: the E-PLURIBUS Study. JACC Cardiovasc Imaging. 2022;S1936–878X(22)00490–9.
Requena-Ibáñez JA, Santos-Gallego CG, Rodriguez Cordero AJ, et al. Not only how much, but also how to, when measuring epicardial adipose tissue. Magn Reson Imaging. 2022;86:149–51.
Funding
This study has been funded by an Investigator-Initiated Grant to Dr. Badimon by Lexicon Pharmaceuticals, Inc. Dr Requena-Ibañez was sup- ported by a fellowship from La Caixa Foundation (ID 100010434). The fellowship code is LCF/BQ/EU21/11890141.
Author information
Authors and Affiliations
Contributions
All authors have read, approved, and contributed equally to the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants are in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The protocol was approved by the Mount Sinai Institutional Review Board (GCO 22–0574) and received IND 165123 by the FDA.
Consent to Participate
Informed consent is obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Competing Interests
This study has been funded by an Investigator-Initiated Grant to Dr. Badimon by Lexicon Pharmaceuticals, Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Drs. Soto Pérez and Rodríguez-Capitán share the first authorship
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pérez, M.S., Rodríguez-Capitán, J., Requena-Ibáñez, J.A. et al. Rationale and Design of the SOTA-P-CARDIA Trial (ATRU-V): Sotagliflozin in HFpEF Patients Without Diabetes. Cardiovasc Drugs Ther (2023). https://doi.org/10.1007/s10557-023-07469-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-023-07469-6