Abstract
Purpose
Treatment of atherosclerotic rabbits with intravenous methotrexate or etoposide carried in lipid nanoemulsions (LDE) markedly reduced the lesions in the aorta. Here, the combined treatment with LDE-methotrexate and LDE-etoposide was investigated aiming to increase the anti-atherosclerosis effect.
Methods
Thirty-six male rabbits received a diet with 1 % cholesterol for 2 months. After the first month, the animals received 4 weekly intravenous injections of LDE-methotrexate (4 mg/kg dose), LDE-etoposide (6 mg/kg), or a combination of those two drugs, while the control animals were injected with LDE (n = 9 for each group).
Results
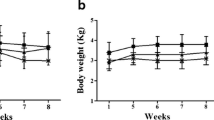
LDE-methotrexate+LDE-etoposide reduced aortic lesion areas by 95 % compared with controls and the intima-media ratio was reduced five-fold, whereas LDE-methotrexate reduced the lesions by 81 % and LDE-etoposide by 83 %. Compared to controls, the positive area of macrophages and MMP-9 in the arterial intima was significantly reduced in all treated groups (p < 0.001), but the MMP9 reduction was greater with the combined chemotherapy than the reduction achieved by the isolated treatments. Presence of CD3 positive cells was equal in controls and LDE-methotrexate+LDE-etoposide treated animals. However, FOXP3 positive T lymphocytes in the intima were increased in the LDE-methotrexate+LDE-etoposide rabbits. Weight, food intake evolution and the hematologic parameters suggested that the treatment had very low toxicity.
Conclusions
Compared to the single treatments with LDE-methotrexate and LDE-etoposide, the combined treatment was more effective in reducing the atherosclerotic lesions. Because the toxicity of the novel drug-target combined scheme was low, those results favor the possibility of future clinical studies in patients with cardiovascular disease.





Similar content being viewed by others
References
Charo IF, Taub R. Anti-inflammatory therapeutics for the treatment of atherosclerosis. Nat Rev Drug Discov. 2011;10:365–76.
Ross R. Atherosclerosis - an inflammatory disease. N Engl J Med. 1999;14:115–26.
Maranhão RC, Roland IA, Toffoletto O, Ramires JA, Gonçalves RP, Mesquita CH. Plasma kinetic behavior in hyperlipidemic subjects of a lipidic microemulsion that binds to low density lipoprotein receptors. Lipids. 1997;32:627–33.
Maranhao RC, Tavares ER, Padoveze AF, Valduga CJ, Rodrigues DG, Pereira MD. Paclitaxel associated with cholesterol-rich nanoemulsions promotes atherosclerosis regression in the rabbit. Atherosclerosis. 2008;197:959–66.
Tavares ER, Freitas FR, Diament J, Maranhão RC. Reduction of atherosclerotic lesions in rabbits treated with etoposide associated with cholesterol-rich nanoemulsions. Int J Nanomedicine. 2011;6:2297–304.
Bulgarelli AC, Leite Jr AC, Dias AA, Maranhão RC. Anti-atherogenic effects of methotrexate carried by a lipid nanoemulsion that binds to LDL receptors in cholesterol-fed rabbits. Cardiovasc Drugs Ther. 2013;27:531–9.
Valduga CJ, Fernandes DC, Lo Prete AC, Azevedo CHM, Rodrigues DG, Maranhao RC. Use of a cholesterol-rich microemulsion that binds to low-density lipoprotein receptors as vehicle for etoposide. J Pharm Pharmacol. 2003;55:1615–22.
Dias ML, Carvalho JP, Rodrigues DG, Graziani SR, Maranhão RC. Pharmacokinetics and tumor uptake of a derivatized form of paclitaxel associated to a cholesterol rich nanoemulsion in patients with gynecologic cancers. Cancer Chemother Pharmacol. 2007;59:105–11.
Maranhão RC, Graziani SR, Yamaguchi N, Melo RF, Latrilha MC, Rodrigues DG, et al. Association of carmustine with a lipid emulsion: in vitro, in vivo and preliminary studies in cancer patients. Cancer Chemother Pharmacol. 2002;49:487–98.
Ginsburg GS, Small DM, Atkinson D. Microemulsion of phospholipids and cholesterol esters Protein-free models of low density lipoprotein. J Biol Chem. 1982;257:8216–27.
Maranhão RC, César TB, Pedroso-Mariani SR, Hirata MH, Mesquita CH. Metabolic behavior in rats of a nonprotein microemulsion resembling low density lipoprotein. Lipids. 1993;28:691–6.
Rosowsky A, Forsch RA, Yu CS, Lazarus H, Beardsley GP. Methotrexate analogues. Divergent influence of alkyl chain on the dihydrofolate reductase affinity and cytotoxicity of methotrexate monoesters. J Med Chem. 1984;27:605–9.
Hall TC, Roberts D, Kessel DH. Methotrexate and folic reductase in human cancer. Eur J Cancer. 1996;2:135–42.
Bonney RJ, Maley F. Effect of methotrexate on thymidylate synthetase in cultured parenchymal cells isolated from regenerating rat liver. Cancer Res. 1975;8:1950–6.
Engel R, Valkov NI, Gump JL, Hazlehurst L, Dalton WS, Sullivan DM. The cytoplasmic trafficking of DNA topoisomerase II alpha correlates with etoposide resistance in human myeloma cells. Exp Cell Res. 2004;295:421–31.
Wessels JA, Huizinga TW, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford). 2008;47:249–55.
Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: implications for the treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007;65:168–73.
Bulgarelli A, Martins Dias AA, Caramelli B, Maranhão RC. Treatment with methotrexate inhibits atherogenesis in cholesterol-fed rabbits. J Cardiovasc Pharmacol. 2012;59:308–14.
de la Llera-Moya M, Rothblat GH, Glick JM, England JM. Etoposide treatment suppresses atherosclerotic plaque development in cholesterol-fed rabbits. Arterioscler Thromb. 1992;12:1363–70.
Esparza L, De Haro J, Bleda S, Acin F. Non-Fas (CD95/APO1)-mediated apoptosis of activated T cells inhibits thedevelopment of atherosclerosis. Interact Cardiovasc Thorac Surg. 2012;15:340–3.
Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;3:251–62.
Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1996;6:131–8.
de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, van der Wal AC. Low numbers of foxp3 positive regulatory t cells are present in all developmental stages of human atherosclerotic lesions. PLoS One. 2007;2(8):e779.
Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT). J Thromb Haemost. 2009;7 Suppl 1:332–9.
Acknowledgments
This work was financially supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), São Paulo, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasília, Brazil. Dr Maranhão has a Research Award from CNPq. We thank Maria das Dores for technical support.
Conflict of Interest
The authors report no conflicts of interest in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leite, A.C.A., Solano, T.V., Tavares, E.R. et al. Use of Combined Chemotherapy with Etoposide and Methotrexate, both Associated to Lipid Nanoemulsions for Atherosclerosis Treatment in Cholesterol-fed Rabbits. Cardiovasc Drugs Ther 29, 15–22 (2015). https://doi.org/10.1007/s10557-014-6566-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-014-6566-1