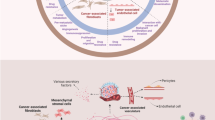
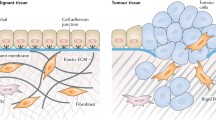
Abstract
Current cancer research focuses mainly upon the cancer cells in malignant tumours and is providing a growing database about aberrations in their genetic composition. However, tumours also contain non-cancerous host tissue, referred to as the stroma, which plays an active and indispensable role in tumour growth and influences the virulence of the neoplasm towards the host. Many cell types inhabit the stroma, amidst apparently inert fibrous and viscous matrix material, composed of complex polysaccharides, proteins and other molecules. Actually, all of these elements are in constant turnover, causing unpredictable evolution in the properties of the community. This article provides pathologic observations and data on reciprocal interactions between these stromal and neoplastic components of tumours and how they change during the course of the disease. Malignant progression depends upon dauntingly intricate communications between different specialised lineages within the cellular society, which enable rapid adaptation to changing circumstances. Opportunistic misuse of such communication networks enables tumour cells to recruit and incorporate adjacent normal stroma into their midst, so that they may grow, infiltrate and parasitise the host. The absolute dependency of primary tumours and metastases on their diverse stromal components for survival and their insatiable need to continuously recruit more stroma to support expansion, renders them vulnerable to strategies capable of disrupting the cellular interactions involved. This dependency is of critical importance for cancer therapy research, and proposed methods for turning this parasitic behaviour of tumours against themselves are suggested below.

Similar content being viewed by others
References
Folkman, J. (2006). Angiogenesis. Annual Review of Medicine, 57, 1–18.
Yamagiwa, K., Ichikawa, K., cited by Yamagiwa KaI, K. (1918). Experimental study of the pathogenesis of carcinoma. Journal of Cancer Research 3, 1–29.
Orr, J. W. (1938). The changes antecedent to tumour formation during the treatment of mouse skin with carcinogenic hydrocarbons. Journal of Pathology and Bacteriology, 46, 495–515.
Billingham, R. E., Orr, J. W., & Woodhouse, D. L. (1951). Transplantation od skin components during chemical carcinogenesis with 20-methylcholanthrene. British Journal of Cancer, 5, 417–432.
Marchant, J., & Orr, J. W. (1953). Further attempts to analyse the role of epidermis and deeper tissues in experimental chemical carcinogenesis by transplantation and other method. British Journal of Cancer, 7, 329–341.
Orr, J. W., & Spencer, A. T. (1972). Transplantation studies of the role of the stroma in epidermal carcinogenesis. In D. Tarin (Ed.), Tissue interactions in carcinogenesis (pp. 291–303). London: Academic.
Tarin, D. (2012). Inappropriate gene expression in human cancer and its far-reaching biological and clinical significance. Cancer Metastasis Reviews, 31, 21–39.
Pelosof, L. C., & Gerber, D. E. (2010). Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clinic Proceedings Mayo Clinic, 85, 838–854.
Darnell, R., & Posner, J. (2011). Paraneoplastic syndromes. Oxford: Oxford University Press.
Spemann, H. (1938). Embryonic development and induction. New Haven: Yale University Press.
Grobstein, C. (1967). Mechanisms of organogenetic tissue interaction. National Cancer Institute Monograph, 26, 279–299.
Saxén, L. (1972). Interactive mechanisms in morphogenesis. In D. Tarin (Ed.), Tissue interactions in carcinogenesis (pp. 49–80). London: Academic.
Kratchwil, K. (2001). Epithelial mesenchymal interactions. http://onlinelibrarywileycom/doi/101038/npgels0001141/full. Accessed 12 April 2013
Franks, T. J., Colby, T. V., Travis, W. D., et al. (2008). Resident cellular components of the human lung: current knowledge and goals for research on cell phenotyping and function. Proceedings of the American Thoracic Society, 5, 763–766.
Beers, M. F., & Morrisey, E. E. (2011). The three R’s of lung health and disease: repair, remodeling, and regeneration. The Journal of Clinical Investigation, 121, 2065–2073.
Cardoso, W. V., & Lu, J. (2006). Regulation of early lung morphogenesis: questions, facts and controversies. Development, 133, 1611–1624.
Cardoso, W. V., & Whitsett, J. A. (2008). Resident cellular components of the lung: developmental aspects. Proceedings of the American Thoracic Society, 5, 767–771.
Grobstein, C. (1953). Morphogenetic interaction between embryonic mouse tissues separated by a membrane filter. Nature, 172, 869–870.
Kratochwil, K. (1972). Tissue interaction during embryonic development. In D. Tarin (Ed.), Tissue interactions in carcinogenesis (pp. 1–47). London: Academic.
Millar, S. E. (2002). Molecular mechanisms regulating hair follicle development. The Journal of Investigative Dermatology, 118, 216–225.
Zorn, A.M. (2008). Liver development. In StemBook. Cambridge, MA. http://www.ncbi.nlm.nih.gov/books/NBK27068/pdf/Liver_development.pdf. Accessed 12 April 2013
Landsman, L., Nijagal, A., Whitchurch, T. J., et al. (2011). Pancreatic mesenchyme regulates epithelial organogenesis throughout development. PLoS Biology, 9, e1001143.
Tabin, C., & Wolpert, L. (2007). Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes & Development, 21, 1433–1442.
Hölldobler, B., & Wilson, E. (2008). The superorganism: the beauty, elegance, and strangeness of insect societies. New York: W.W. Norton Inc.
Corning, P. (2002). The re-emergence of emergence: a venerable concept in search of a theory. Complexity, 7, 18–30.
Arp, R. (2008). Life and the homeostatic organization view of biological phenomena. Cosmos and History: The Journal of Natural and Social Philosophy, 4, 260–282.
Tarin, D. (2011). Cell and tissue interactions in carcinogenesis and metastasis and their clinical significance. Seminars in Cancer Biology, 21, 72–82.
Meilhac, S. M., Adams, R. J., Morris, S. A., et al. (2009). Active cell movements coupled to positional induction are involved in lineage segregation in the mouse blastocyst. Developmental Biology, 331, 210–221.
Rinn, J. L., Bondre, C., Gladstone, H. B., et al. (2006). Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genetics, 2, e119.
Shannon, J. M., & Hyatt, B. A. (2004). Epithelial–mesenchymal interactions in the developing lung. Annual Review of Physiology, 66, 625–645.
Billingham, R. E., & Silvers, W. K. (1963). The origin and conservation of epidermal specificities. The New England Journal of Medicine, 268, 539–545. concl.
Billingham, R., & Silvers, W. (1968). Dermoepidermal interactions and epithelial specificity. In R. Fleischmajer & R. Billingham (Eds.), Epithelial–mesenchymal interactions (pp. 252–266). Baltimore: Williams and Wilkins.
Cunha, G. R., Fujii, H., Neubauer, B. L., et al. (1983). Epithelial–mesenchymal interactions in prostatic development. I. Morphological observations of prostatic induction by urogenital sinus mesenchyme in epithelium of the adult rodent urinary bladder. The Journal of Cell Biology, 96, 1662–1670.
Cunha, G. R., Hayward, S. W., & Wang, Y. Z. (2002). Role of stroma in carcinogenesis of the prostate. Differentiation, 70, 473–485.
Dawe, C. (1972). Epithelial–mesenchymal interactions in relation to the genesis of polyoma virus-induced tumors of mouse salivary gland. In D. Tarin (Ed.), Tissue interactions in carcinogenesis (pp. 305–358). London: Academic.
Tarin, D., Price, J. E., Kettlewell, M. G., et al. (1984). Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunts. Cancer Research, 44, 3584–3592.
Suzuki, M., Mose, E. S., Montel, V., et al. (2006). Dormant cancer cells retrieved from metastasis-free organs regain tumorigenic and metastatic potency. The American Journal of Pathology, 169, 673–681.
Paget, S. (1889). The distribution of secondary growths in cancer of the breast. The Lancet, i, 571–573.
Hart, I. R., & Fidler, I. J. (1980). Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Research, 40, 2281–2287.
Goodison, S., Kawai, K., Hihara, J., et al. (2003). Prolonged dormancy and site-specific growth potential of cancer cells spontaneously disseminated from nonmetastatic breast tumors as revealed by labeling with green fluorescent protein. Clinical Cancer Research, 9, 3808–3814.
Bresalier, R. S., Raper, S. E., Hujanen, E. S., et al. (1987). A new animal model for human colon cancer metastasis. International Journal of Cancer, 39, 625–630.
Morikawa, K., Walker, S. M., Nakajima, M., et al. (1988). Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Research, 48, 6863–6871.
Naito, S., von Eschenbach, A. C., Giavazzi, R., et al. (1986). Growth and metastasis of tumor cells isolated from a human renal cell carcinoma implanted into different organs of nude mice. Cancer Research, 46, 4109–4115.
Stephenson, R. A., Dinney, C. P., Gohji, K., et al. (1992). Metastatic model for human prostate cancer using orthotopic implantation in nude mice. Journal of the National Cancer Institute, 84, 951–957.
Montel, V., Mose, E. S., & Tarin, D. (2006). Tumor–stromal interactions reciprocally modulate gene expression patterns during carcinogenesis and metastasis. International Journal of Cancer Journal International du Cancer, 119, 251–263.
Bao, L., Pigott, R., Matsumura, Y., et al. (1993). Correlation of VLA-4 integrin expression with metastatic potential in various human tumour cell lines. Differentiation, 52, 239–246.
Tarin, D., & Croft, C. B. (1970). Ultrastructural studies of wound healing in mouse skin. II. Dermo-epidermal interrelationships. Journal of Anatomy, 106, 79–91.
Croft, C. B., & Tarin, D. (1970). Ultrastructural studies of wound healing in mouse skin. I. Epithelial behaviour. Journal of Anatomy, 106, 63–77.
Cowell, T. (1972). Control of epithelial invasion by connective tissue during embedding of the mouse ovum. In D. Tarin (Ed.), Tissue interactions in carcinogenesis. London: Academic.
Konijeti, R., Rajfer, J., & Askari, A. (2009). Placenta percreta and the urologist. Reviews in Urology, 11, 173–176.
Tarin, D. (1968). Further electron microscopic studies on the mechanism of carcinogenesis: the specificity of the changes in carcinogen-treated mouse skin. International Journal of Cancer Journal International du Cancer, 3, 734–742.
Tarin, D. (1969). Fine structure of murine mammary tumours: the relationship between epithelium and connective tissue in neoplasms induced by various agents. British Journal of Cancer, 23, 417–425.
Tarin, D. (1972). Morphological studies on the mechanism of carcinogenesis. In D. Tarin (Ed.), Tissue interactions in carcinogenesis (pp. 227–289). London: Academic.
Brand, K. G., Buoen, L. C., Johnson, K. H., et al. (1975). Etiological factors, stages, and the role of the foreign body in foreign body tumorigenesis: a review. Cancer Research, 35, 279–286.
Buoen, L. C., Brand, I., & Brand, K. G. (1975). Foreign-body tumorigenesis: in vitro isolation and expansion of preneoplastic clonal cell populations. Journal of the National Cancer Institute, 55, 721–723.
Karp, R. D., Johnson, K. H., Buoen, L. C., et al. (1973). Tumorigenesis by Millipore filters in mice: histology and ultrastructure of tissue reactions as related to pore size. Journal of the National Cancer Institute, 51, 1275–1285.
Tarin, D. (2012). Clinical and biological implications of the tumor microenvironment. Cancer Microenvironment, 5, 95–112.
Thiery, J. P. (2002). Epithelial–mesenchymal transitions in tumour progression. Nature Reviews. Cancer, 2, 442–454.
Kalluri, R., & Weinberg, R. A. (2009). The basics of epithelial–mesenchymal transition. The Journal of Clinical Investigation, 119, 1420–1428.
Mani, S. A., Guo, W., Liao, M. J., et al. (2008). The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell, 133, 704–715.
Polyak, K., & Weinberg, R. A. (2009). Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature Reviews. Cancer, 9, 265–273.
Thompson, E. W., Newgreen, D. F., & Tarin, D. (2005). Carcinoma invasion and metastasis: a role for epithelial–mesenchymal transition? Cancer Research, 65, 5991–5995. discussion 5995.
Kang, Y., & Massague, J. (2004). Epithelial–mesenchymal transitions: twist in development and metastasis. Cell, 118, 277–279.
Zeng, Q., Li, W., Lu, D., et al. (2012). CD146, an epithelial–mesenchymal transition inducer, is associated with triple-negative breast cancer. Proceedings of the National Academy of Sciences of the United States of America, 109, 1127–1132.
Trimboli, A. J., Fukino, K., de Bruin, A., et al. (2008). Direct evidence for epithelial–mesenchymal transitions in breast cancer. Cancer Research, 68, 937–945.
Thiery, J. P., Acloque, H., Huang, R. Y., et al. (2009). Epithelial–mesenchymal transitions in development and disease. Cell, 139, 871–890.
Trelstad, R. L., Hay, E. D., & Revel, J. D. (1967). Cell contact during early morphogenesis in the chick embryo. Developmental Biology, 16, 78–106.
Hay, E. (1968). Organization and fine structure of epithelium and mesenchyme in the developing chick embryo. In R. Fleischmajer & R. Billingham (Eds.), Epithelial mesenchymal interactions. Baltimore: Williams and Wilkins.
Yang, J., & Weinberg, R. A. (2008). Epithelial–mesenchymal transition: at the crossroads of development and tumor metastasis. Developmental Cell, 14, 818–829.
Tarin, D. (1971). Histological features of neural induction in Xenopus laevis. Journal of Embryology and Experimental Morphology, 26, 543–570.
Tarin, D. (1971). Scanning electron microscopical studies of the embryonic surface during gastrulation and neurulation in Xenopus laevis. Journal of Anatomy, 109, 535–547.
Tarin, D. (1972). Ultrastructural features of neural induction in Xenopus laevis. Journal of Anatomy, 111, 1–28.
Tarin, D., & Sturdee, A. P. (1971). Early limb development of Xenopus laevis. Journal of Embryology and Experimental Morphology, 26, 169–179.
Tarin, D., & Sturdee, A. P. (1974). Ultrastructural features of ectodermal–mesenchymal relationships in the developing limb of Xenopus laevis. Journal of Embryology and Experimental Morphology, 31, 287–303.
Toivonen, S., Tarin, D., & Saxen, L. (1976). The transmission of morphogenetic signals from amphibian mesoderm to ectoderm in primary induction. Differentiation, 5, 49–55.
Tarin, D. (2005). The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Research, 65, 5996–6000.
Gise, A., & Pu, W. T. (2012). Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circulation Research, 110, 1628–1645.
Korsching, E., Packeisen, J., Liedtke, C., et al. (2005). The origin of vimentin expression in invasive breast cancer: epithelial–mesenchymal transition, myoepithelial histogenesis or histogenesis from progenitor cells with bilinear differentiation potential? The Journal of Pathology, 206, 451–457.
Gilbert, S. (2000). Developmental biology (6th edition ed). Sinauer Associates: Sunderland, MA, Chapter 14: Intermediate Mesoderm section Available from: http://www.ncbi.nlm.nih.gov/books/NBK10089/.
Sucheston, M. E., & Cannon, M. S. (1968). Development of zonular patterns in the human adrenal gland. Journal of Morphology, 126, 477–491.
Kempna, P., & Fluck, C. E. (2008). Adrenal gland development and defects. Best Practice & Research. Clinical Endocrinology & Metabolism, 22, 77–93.
Satoh, M. (1991). Histogenesis and organogenesis of the gonad in human embryos. Journal of Anatomy, 177, 85–107.
Kalluri, R., & Neilson, E. G. (2003). Epithelial–mesenchymal transition and its implications for fibrosis. The Journal of Clinical Investigation, 112, 1776–1784.
Osterreicher, C. H., Penz-Osterreicher, M., Grivennikov, S. I., et al. (2011). Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proceedings of the National Academy of Sciences of the United States of America, 108, 308–313.
Humphreys, B. D., Lin, S. L., Kobayashi, A., et al. (2010). Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. The American Journal of Pathology, 176, 85–97.
Tomaskovic-Crook, E., Thompson, E. W., & Thiery, J. P. (2009). Epithelial to mesenchymal transition and breast cancer. Breast Cancer Research, 11, 213.
May, C. D., Sphyris, N., Evans, K. W., et al. (2011). Epithelial–mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progression. Breast Cancer Research, 13, 202.
Yang, J., Mani, S. A., Donaher, J. L., et al. (2004). Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell, 117, 927–939.
Chui, M. H. (2012). Insights into cancer metastasis from a clinicopathologic perspective: epithelial mesenchymal transition is not a necessary step. International Journal of Cancer Journal International du Cancer. doi:10.1002/ijc.27745.
Cardiff, R. D. (2010). The pathology of EMT in mouse mammary tumorigenesis. Journal of Mammary Gland Biology and Neoplasia, 15, 225–233.
Hennessy, B. T., Gonzalez-Angulo, A. M., Stemke-Hale, K., et al. (2009). Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Research, 69, 4116–4124.
Taube, J. H., Herschkowitz, J. I., Komurov, K., et al. (2010). Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proceedings of the National Academy of Sciences of the United States of America, 107, 15449–15454.
Brabletz, T. (2012). To differentiate or not—routes towards metastasis. Nature Reviews. Cancer, 12, 425–436.
Brabletz, T., Jung, A., Reu, S., et al. (2001). Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proceedings of the National Academy of Sciences of the United States of America, 98, 10356–10361.
Kubiak, R., & Szadowska, A. (1997). Invasive lobular carcinoma: correlations between morphological features, vimentin expression, oestrogen receptor status and prognosis. The Breast, 6, 89–96.
Armstrong, A. J., Marengo, M. S., Oltean, S., et al. (2011). Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Molecular Cancer Research, 9, 997–1007.
Powell, A. A., Talasaz, A. H., Zhang, H., et al. (2012). Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS One, 7, e33788.
Sun, Y., Campisi, J., Higano, C., et al. (2012).Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nature Medicine 18, 1359–1368.
O’Mahony, F. C., Faratian, D., Varley, J., et al. (2012). The use of automated quantitative analysis to evaluate epithelial-to-mesenchymal transition associated proteins in clear cell renal cell carcinoma. PLoS One, 7, e31557.
Leroy, P., & Mostov, K. E. (2007). Slug is required for cell survival during partial epithelial–mesenchymal transition of HGF-induced tubulogenesis. Molecular Biology of the Cell, 18, 1943–1952.
Ledford, H. (2011). Cancer theory faces doubts. Nature, 472, 273.
Hugo, H., Ackland, M. L., Blick, T., et al. (2007). Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. Journal of Cellular Physiology, 213, 374–383.
Christiansen, J. J., & Rajasekaran, A. K. (2006). Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Research, 66, 8319–8326.
Zhou, H., Wu, S., Joo, J. Y., et al. (2009). Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell, 4, 381–384.
Wilmut, I., Schnieke, A. E., McWhir, J., et al. (1997). Viable offspring derived from fetal and adult mammalian cells. Nature, 385, 810–813.
Chaffer, C. L., Thompson, E. W., & Williams, E. D. (2007). Mesenchymal to epithelial transition in development and disease. Cells, Tissues, Organs, 185, 7–19.
Tarin, D. (1967). Sequential electron microscopical study of experimental mouse skin carcinogenesis. International Journal of Cancer, 2, 195–211.
Tarin, D. (1976). Cellular interactions in neoplasia. In L. Weiss (Ed.), Fundamental aspects of metastasis (pp. 151–187). Amsterdam: North Holland Publishing Co.
Sugino, T., Gorham, H., Yoshida, K., et al. (1996). Progressive loss of CD44 gene expression in invasive bladder cancer. The American Journal of Pathology, 149, 873–882.
Viadana, E., Bross, I. D., & Pickren, J. W. (1973). An autopsy study of some routes of dissemination of cancer of the breast. British Journal of Cancer, 27, 336–340.
Noltenius, C., & Noltenius, H. (1985). Dormant tumor cells in liver and brain. An autopsy study on metastasizing tumors. Pathology Research and Practice, 179, 504–511.
Suzuki, M., Mose, E., Galloy, C., et al. (2007). Osteopontin gene expression determines spontaneous metastatic performance of orthotopic human breast cancer xenografts. The American Journal of Pathology, 171, 682–692.
Urquidi, V., Sloan, D., Kawai, K., et al. (2002). Contrasting expression of thrombospondin-1 and osteopontin correlates with absence or presence of metastatic phenotype in an isogenic model of spontaneous human breast cancer metastasis. Clinical Cancer Research, 8, 61–74.
Agrawal, D., Chen, T., Irby, R., et al. (2002). Osteopontin identified as lead marker of colon cancer progression, using pooled sample expression profiling. Journal of the National Cancer Institute, 94, 513–521.
Ang, C., Chambers, A. F., Tuck, A. B., et al. (2005). Plasma osteopontin levels are predictive of disease stage in patients with transitional cell carcinoma of the bladder. BJU International, 96, 803–805.
Donati, V., Boldrini, L., Dell’Omodarme, M., et al. (2005). Osteopontin expression and prognostic significance in non-small cell lung cancer. Clinical Cancer Research, 11, 6459–6465.
Jang, T., Savarese, T., Low, H. P., et al. (2006). Osteopontin expression in intratumoral astrocytes marks tumor progression in gliomas induced by prenatal exposure to N-ethyl-N-nitrosourea. The American Journal of Pathology, 168, 1676–1685.
Matusan, K., Dordevic, G., Stipic, D., et al. (2006). Osteopontin expression correlates with prognostic variables and survival in clear cell renal cell carcinoma. Journal of Surgical Oncology, 94, 325–331.
Roland, P. Y., Kelly, F. J., Kulwicki, C. Y., et al. (2004). The benefits of a gynecologic oncologist: a pattern of care study for endometrial cancer treatment. Gynecologic Oncology, 93, 125–130.
Wai, P. Y., & Kuo, P. C. (2004). The role of osteopontin in tumor metastasis. The Journal of Surgical Research, 121, 228–241.
Feng, W., McCabe, N. P., Mahabeleshwar, G. H., et al. (2008). The angiogenic response is dictated by beta3 integrin on bone marrow-derived cells. The Journal of Cell Biology, 183, 1145–1157.
Kelly, P. N., Dakic, A., Adams, J. M., et al. (2007). Tumor growth need not be driven by rare cancer stem cells. Science, 317, 337.
Price, J. E., Syms, A. J., Wallace, J. S., et al. (1986). Cellular immortality, clonogenicity, tumorigenicity and the metastatic phenotype. European Journal of Cancer & Clinical Oncology, 22, 349–355.
Visvader, J. E., & Lindeman, G. J. (2008). Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature Reviews. Cancer, 8, 755–768.
Wang, G. X., Zhan, Y. A., Hu, H. L., et al. (2012). Mesenchymal stem cells modified to express interferon-beta inhibit the growth of prostate cancer in a mouse model. The Journal of International Medical Research, 40, 317–327.
Schrodinger, E. (1944). What is life (p. 194). Cambridge, UK: Cambridge University Press.
Acknowledgments
The author acknowledges with gratitude the valuable comments, suggestions and discussions provided by G. G. Miklos PhD and D. L. Darling MD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tarin, D. Role of the host stroma in cancer and its therapeutic significance. Cancer Metastasis Rev 32, 553–566 (2013). https://doi.org/10.1007/s10555-013-9438-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-013-9438-4