Abstract
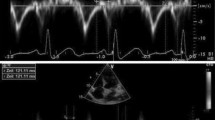
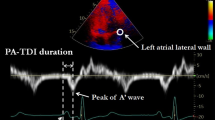
Identifying patients at high risk of atrial fibrillation (AF) recurrence remains challenging. This study aimed to evaluate total atrial conduction time (TACT) and left atrial (LA) asynchrony as predictors of AF recurrence. Consecutive patients after the first AF episode, terminated either spontaneously or with cardioversion, underwent transthoracic echocardiography. TACT, estimated by the time delay between the onset of P-wave and the peak A′-wave on the Tissue Doppler Imaging (PA-TDI duration), atrial volumetric and functional parameters, and biatrial strain were assessed. We calculated mean PA-TDI—the average of PA-TDI measurements in all left atrial (LA) walls—and the difference between the longest and the shortest PA interval (DLS) and the standard deviation of 4 PA intervals (SD4) to assess the LA global remodeling and asynchrony, respectively. The primary endpoint was AF recurrence. Patients with recurrent AF had significantly prolonged PA-TDI intervals in each LA wall—and thus mean PA-TDI—than those without recurrence (mean PA-TDI: 157.4 ± 17.9 vs. 110.2 ± 7.7 ms, p < 0.001). At univariate analysis, LA maximum volume index, total LA emptying fraction, right atrial maximum volume index, PA-TDI, DLS, and SD4 were predictors of AF recurrence. At multivariable analysis, PA-TDI intervals in all LA walls remained strong predictors with mean PA-TDI (odds ratio 1.04; 95% confidence interval 1.03–1.06) having an optimal cutoff of 125.8 ms in receiver operator characteristics curve analysis providing 98% sensitivity and 100% specificity for AF recurrence (area under the curve = 0.989). PA-TDI was an independent predictor of AF recurrence and outperformed established echocardiographic parameters.


Similar content being viewed by others
Code availability
R code available upon request.
Data availability
Data available upon request
References
Conen D (2018) Epidemiology of atrial fibrillation. Eur Heart J 39(16):1323–1324. https://doi.org/10.1093/eurheartj/ehy171
Kirchhof P, Benussi S, Kotecha D et al (2016) 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 37(38):2893–2962. https://doi.org/10.1093/eurheartj/ehw210
Haim M, Hoshen M, Reges O, Rabi Y, Balicer R, Leibowitz M (2015) Prospective national study of the prevalence, incidence, management and outcome of a large contemporary cohort of patients with incident non-valvular atrial fibrillation. J Am Heart Assoc. 4(1):1–12. https://doi.org/10.1161/JAHA.114.001486
Wolf PA, Abbott RD, Kannel WB (1991) Atrial fibrillation as an independent risk factor for stroke: the framingham study. Stroke. https://doi.org/10.1161/01.STR.22.8.983
White H, Boden-Albala B, Wang C et al (2005) Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the northern Manhattan study. Circulation 111(10):1327–1331. https://doi.org/10.1161/01.CIR.0000157736.19739.D0
Ganesan AN, Shipp NJ, Brooks AG et al (2013) Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2(2):e004549. https://doi.org/10.1161/JAHA.112.004549
Hirose T, Kawasaki M, Tanaka R et al (2012) Left atrial function assessed by speckle tracking echocardiography as a predictor of new-onset non-valvular atrial fibrillation: results from a prospective study in 580 adults. Eur Heart J Cardiovasc Imaging. 13(3):243–250. https://doi.org/10.1093/ejechocard/jer251
Blume GG, McLeod CJ, Barnes ME et al (2011) Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr 12(6):421–430. https://doi.org/10.1093/ejechocard/jeq175
Abecasis J, Dourado R, Ferreira A et al (2009) Left atrial volume calculated by multi-detector computed tomography may predict successful pulmonary vein isolation in catheter ablation of atrial fibrillation. Europace 11(10):1289–1294. https://doi.org/10.1093/europace/eup198
Weijs B, De Vos CB, Tieleman RG et al (2011) Clinical and echocardiographic correlates of intra-atrial conduction delay. Europace 13(12):1681–1687. https://doi.org/10.1093/europace/eur261
Merckx KL, De Vos CB, Palmans A et al (2005) Atrial activation time determined by transthoracic doppler tissue imaging can be used as an estimate of the total duration of atrial electrical activation. J Am Soc Echocardiogr 18(9):940–944. https://doi.org/10.1016/j.echo.2005.03.022
Erdem FH, Erdem A, Özlü F et al (2016) Electrophysiological validation of total atrial conduction time measurement by tissue doppler echocardiography according to age and sex in healthy adults. J Arrhythmia 32(2):127–132. https://doi.org/10.1016/j.joa.2015.11.006
De Vos CB, Weijs B, Crijns HJGM et al (2009) Atrial tissue Doppler imaging for prediction of new-onset atrial fibrillation. Heart 95(10):835–840. https://doi.org/10.1136/hrt.2008.148528
Löbe S, Knopp H, Le TV et al (2018) Left atrial asynchrony measured by pulsed-wave tissue doppler is associated with abnormal atrial voltage and recurrences of atrial fibrillation after catheter ablation. JACC Clin Electrophysiol 4(12):1640–1641. https://doi.org/10.1016/j.jacep.2018.08.017
Den Uijl DW, Gawrysiak M, Tops LF et al (2011) Prognostic value of total atrial conduction time estimated with tissue Doppler imaging to predict the recurrence of atrial fibrillation after radiofrequency catheter ablation. Europace 13(11):1533–1540. https://doi.org/10.1093/europace/eur186
Dinov B, Knopp H, Löbe S et al (2016) Patterns of left atrial activation and evaluation of atrial dyssynchrony in patients with atrial fibrillation and normal controls: factors beyond the left atrial dimensions. Hear Rhythm. 13(9):1829–1836. https://doi.org/10.1016/j.hrthm.2016.06.003
Dilaveris PE, Gialafos EJ, Sideris SK et al (1998) Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J 135(5):733–738. https://doi.org/10.1016/s0002-8703(98)70030-4
Hatam N, Aljalloud A, Mischke K et al (2014) Interatrial conduction disturbance in postoperative atrial fibrillation: a comparative study of P-wave dispersion and Doppler myocardial imaging in cardiac surgery. J Cardiothorac Surg. 9(1):114. https://doi.org/10.1186/1749-8090-9-114
Abou R, Leung M, Tonsbeek AM et al (2017) Effect of aging on left atrial compliance and electromechanical properties in subjects without structural heart disease. Am J Cardiol 120(1):140–147. https://doi.org/10.1016/j.amjcard.2017.03.243
Kuppahally SS, Akoum N, Burgon NS et al (2010) Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging 3(3):231–239. https://doi.org/10.1161/CIRCIMAGING.109.865683
Maffè S, Paffoni P, Dellavesa P et al (2015) Prognostic value of total atrial conduction time measured with tissue doppler imaging to predict the maintenance of sinus rhythm after external electrical cardioversion of persistent atrial fibrillation. Echocardiography. 32(3):420–427. https://doi.org/10.1111/echo.12702
Müller P, Schiedat F, Bialek A et al (2014) Total atrial conduction time assessed by tissue doppler imaging (PA-TDI interval) to predict early recurrence of persistent atrial fibrillation after successful electrical cardioversion. J Cardiovasc Electrophysiol. https://doi.org/10.1111/jce.12306
Chen MS, Marrouche NF, Khaykin Y et al (2004) Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J Am Coll Cardiol 43(6):1004–1009. https://doi.org/10.1016/j.jacc.2003.09.056
Berruezo A, Tamborero D, Mont L et al (2007) Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J 28(7):836–841. https://doi.org/10.1093/eurheartj/ehm027
Schneider C, Malisius R, Krause K et al (2008) Strain rate imaging for functional quantification of the left atrium: atrial deformation predicts the maintenance of sinus rhythm after catheter ablation of atrial fibrillation. Eur Heart J 29(11):1397–1409. https://doi.org/10.1093/eurheartj/ehn168
Vasamreddy CR, Lickfett L, Jayam VK et al (2004) Predictors of recurrence following catheter ablation of atrial fibrillation using an irrigated-tip ablation catheter. J Cardiovasc Electrophysiol. https://doi.org/10.1046/j.1540-8167.2004.03538.x
Mouselimis D, Tsarouchas AS, Pagourelias ED et al (2020) Left atrial strain, intervendor variability, and atrial fibrillation recurrence after catheter ablation: a systematic review and meta-analysis. Hell J Cardiol. https://doi.org/10.1016/j.hjc.2020.04.008
Antoni ML, Bertini M, Atary JZ et al (2010) Predictive value of total atrial conduction time estimated with tissue doppler imaging for the development of new-onset atrial fibrillation after acute myocardial infarction. Am J Cardiol 106(2):198–203. https://doi.org/10.1016/j.amjcard.2010.02.030
Müller P, Ivanov V, Kara K et al (2017) Total atrial conduction time to predict occult atrial fibrillation after cryptogenic stroke. Clin Res Cardiol 106(2):113–119. https://doi.org/10.1007/s00392-016-1029-2
Ntaios G (2020) Embolic stroke of undetermined source. J Am Coll Cardiol 75(3):333. https://doi.org/10.1016/j.jacc.2019.11.024
Kishore A, Vail A, Majid A et al (2014) Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke 45(2):520–526. https://doi.org/10.1161/STROKEAHA.113.003433
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 16(3):233–271. https://doi.org/10.1093/ehjci/jev014
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical approval
Approved by the Institutional Review Board of AHEPA Hospital and Aristotle University Ethics Committee and conducted according to the current version of the Declaration of Helsinki (2013).
Informed consent
All subjects gave their informed consent prior to their inclusion in the study. All the authors gave consent to submit for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
All patients were imaged in left lateral decubitus position and the leads were placed in as possible identical positions. A 2D echocardiogram, including M-mode, Doppler (PW, CW and color) and TDI echocardiography were obtained in the parasternal short and long axis views.
LA volumes were obtained from the apical views by disc’s method and were indexed to body surface area (BSA) [32]. They were measured at three phases of cardiac cycle: 1. LA maximum volume at the end-systolic phase (just before mitral valve opening), 2. LA minimum volume at the end-diastolic phase (just before mitral valve closure) and 3. Volume before atrial active contraction, LA preA volume obtained from the last frame before mitral valve reopening or at time of the P wave on surface electrocardiography.
The LA function was assessed based on LA volumes by calculating the following equations: 1. Total atrial emptying fraction. 2. Active atrial emptying fraction: LA active ejection fraction, which is considered an index of LA active contraction. 3. Passive atrial emptying fraction, which is considered an index of LA conduit function; and 4. Atrial expansion index: LA expansion index, which is considered an index of LA reservoir function. Left ventricular ejection fraction (LVEF) was calculated from the standard apical 2- and 4-chamber views using the Simpson’s method.
Atrial Strain: The global and regional LA and RA strain was measured by 2D speckle tracking echocardiography (2DSTE). Recordings were processed with acoustic-tracking software (EchoPAC, GE Healthcare), allowing off line semi automated speckle tracking analysis. Along the LA and RA, the endocardium lines were manually traced. An additional epicardial line was generated automatically by the software, creating a region of interest (ROI). The ROI shape was manually adjusted, and the software divided the LA and RA region into six segments and generated the longitudinal strain curve. The zero strain point was set at the beginning of the P wave. Positive peak strain was measured during ventricular systole and negative peak strain during LA systole globally for each of the 12 segments. The total strain was the difference of positive peak strain and negative peak strain. Positive peak strain rate was measured during ventricular systole while early negative peak strain rate was measured during late diastole (see Tables 6, 7, 8, 9, 10, 11).
Rights and permissions
About this article
Cite this article
Karantoumanis, I., Doundoulakis, I., Zafeiropoulos, S. et al. Atrial conduction time associated predictors of recurrent atrial fibrillation. Int J Cardiovasc Imaging 37, 1267–1277 (2021). https://doi.org/10.1007/s10554-020-02113-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-02113-y