Abstract
Purpose
To compare the outcome of allogeneic stem cell transplantation for myeloid malignancies in breast cancer survivors to a contemporaneous control group.
Methods
Medical records of all patients with a history of breast cancer who received allogeneic stem cell transplants at a single, tertiary referral Comprehensive Cancer Center between 2002 and 2019 were reviewed. Transplant outcomes were compared to 289 control patients without a history of breast cancer from the same time period. Main outcomes included survival, disease-free survival, non-relapse mortality, relapse or progression of hematologic malignancy, and incidence of recurrent breast cancer after hematopoietic cell transplantation. Comparisons between women with a history of breast cancer and controls utilized propensity score weighting to balance patient characteristics.
Results
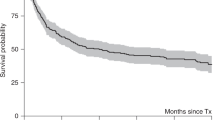
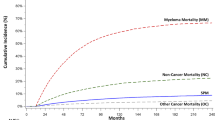
Forty women, ages 30–74 years, with a history of breast cancer received an allogeneic hematopoietic cell transplant for a hematologic malignancy between December 2002 and February 2019. Twelve of the 40 patients are alive with a median survival of 7.4 years (range, 1.9–16.8 years). None of the patients had evidence of recurrent breast cancer prior to death or date of last contact. In multivariable Cox models, all transplant outcomes were similar between the patients and the control group with hematopoietic cell transplant comorbidity score as the most important confounding factor for adjustment in these models.
Conclusion
A history of treated breast cancer should not exclude patients from consideration for allogeneic hematopoietic cell transplantation.


Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
References
DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL (2019) Breast cancer statistics, 2019. CA Cancer J Clin 69(6):438–451
Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) SEER Cancer Statistics Review, 1975–2017. In.: National Cancer Institute, Bethesda, MD, https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site, April 2020.
Lavey RS, Eby NL, Prosnitz LR (1990) Impact of radiation therapy and/or chemotherapy on the risk for a second malignancy after breast cancer. Cancer 66(5):874–881
Curtis RE, Boice JD Jr, Stovall M, Bernstein L, Greenberg RS, Flannery JT, Schwartz AG, Weyer P, Moloney WC, Hoover RN (1992) Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N Engl J Med 326(26):1745–1751
Obedian E, Fischer DB, Haffty BG (2000) Second malignancies after treatment of early-stage breast cancer: lumpectomy and radiation therapy versus mastectomy. J Clin Oncol 18(12):2406–2412
Saso R, Kulkarni S, Mitchell P, Treleaven J, Swansbury GJ, Mehta J, Powles R, Ashley S, Kuan A, Powles T (2000) Secondary myelodysplastic syndrome/acute myeloid leukaemia following mitoxantrone-based therapy for breast carcinoma. Br J Cancer 83(1):91–94
Smith RE, Bryant J, DeCillis A, Anderson S (2003) National Surgical Adjuvant B, Bowel Project E: Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol 21(7):1195–1204
Praga C, Bergh J, Bliss J, Bonneterre J, Cesana B, Coombes RC, Fargeot P, Folin A, Fumoleau P, Giuliani R et al (2005) Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: correlation with doses of epirubicin and cyclophosphamide. J Clin Oncol 23(18):4179–4191
Patt DA, Duan Z, Fang S, Hortobagyi GN, Giordano SH (2007) Acute myeloid leukemia after adjuvant breast cancer therapy in older women: understanding risk. J Clin Oncol 25(25):3871–3876
Kirova YM, De Rycke Y, Gambotti L, Pierga JY, Asselain B, Fourquet A (2008) Institut curie breast cancer study G: second malignancies after breast cancer: the impact of different treatment modalities. Br J Cancer 98(5):870–874
Kirova YM, Gambotti L, De Rycke Y, Vilcoq JR, Asselain B, Fourquet A (2007) Risk of second malignancies after adjuvant radiotherapy for breast cancer: a large-scale, single-institution review. Int J Radiat Oncol Biol Phys 68(2):359–363
Karp JEBA, Visvanathan K, Rugo HS, Moy B, Goldstein LJ, et al (2012) Myelodysplastic syndrome and/or acute myelogenous leukemia (MDS and/or AML) after a breast cancer diagnosis: the National Comprehensive Cancer Network (NCCN) experience. In: Paper presented at the American Association of Cancer Research. https://cancerres.aacrjournals.org/content/72/24_Supplement/S3-5
Burt LM, Ying J, Poppe MM, Suneja G, Gaffney DK (2017) Risk of secondary malignancies after radiation therapy for breast cancer: comprehensive results. Breast 35:122–129
Bazire L, De Rycke Y, Asselain B, Fourquet A, Kirova YM (2017) Risks of second malignancies after breast cancer treatment: long-term results. Cancer Radiother 21(1):10–15
Mayer EL (2013) Early and late long-term effects of adjuvant chemotherapy. Am Soc Clin Oncol 2013:9–14
Miao Y, Everly JJ, Gross TG, Tevar AD, First MR, Alloway RR, Woodle ES (2009) De novo cancers arising in organ transplant recipients are associated with adverse outcomes compared with the general population. Transplantation 87(9):1347–1359
Prentice RL, Kalbfleisch JD, Peterson AV Jr, Flournoy N, Farewell VT, Breslow NE (1978) The analysis of failure times in the presence of competing risks. Biometrics 34(4):541–554
Robins JM, Hernan MA, Brumback B (2000) Marginal structural models and causal inference in epidemiology. Epidemiology 11(5):550–560
Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, Dowsett M et al (2017) 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 377(19):1836–1846
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, Ebert BL, Fenaux P, Larson RA et al (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129(4):424–447
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, Apperley J, Slavin S, Pasquini M, Sandmaier BM et al (2009) Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 15(12):1628–1633
Why, When and Where to Report Conditioning Drugs [https://www.cibmtr.org/Meetings/Materials/CRPDMC/Pages/2015Index.aspx ]. Accessed 11 Feb 2021
AlBugami M, Kiberd B (2014) Malignancies: pre and post transplantation strategies. Transplant Rev (Orlando) 28(2):76–83
Rossi AP, Klein CL (2019) Posttransplant Malignancy. Surg Clin North Am 99(1):49–64
Campistol JM, Cuervas-Mons V, Manito N, Almenar L, Arias M, Casafont F, Del Castillo D, Crespo-Leiro MG, Delgado JF, Herrero JI et al (2012) New concepts and best practices for management of pre- and post-transplantation cancer. Transplant Rev (Orlando) 26(4):261–279
Wong G, Au E, Badve SV, Lim WH (2017) Breast cancer and transplantation. Am J Transplant 17(9):2243–2253
Webster AC, Craig JC, Simpson JM, Jones MP, Chapman JR (2007) Identifying high risk groups and quantifying absolute risk of cancer after kidney transplantation: a cohort study of 15,183 recipients. Am J Transplant 7(9):2140–2151
Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, Chapman JR, Webster AC, Kaldor JM, Grulich AE (2006) Cancer incidence before and after kidney transplantation. JAMA 296(23):2823–2831
Swerdlow SCE, Harris NL et al (2017) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, revised, 4th edn. International Agency for Research on Cancer IARC, Lyon
Lazarus G, Audrey J, Iskandar AWB (2019) Efficacy and safety profiles of programmed cell death-1/programmed cell death ligand-1 inhibitors in the treatment of triple-negative breast cancer: A comprehensive systematic review. Oncol Rev 13(2):425
Bertucci A, Bertucci F, Zemmour C, Lerebours F, Pierga JY, Levy C, Dalenc F, Grenier J, Petit T, Berline M et al (2020) PELICAN-IPC 2015–016/Oncodistinct-003: a prospective, multicenter, open-label, randomized, non-comparative, phase ii study of pembrolizumab in combination with neo adjuvant EC-paclitaxel regimen in HER2-negative inflammatory breast cancer. Front Oncol 10:575978
Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N et al (2020) Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382(9):810–821
Alva AS, Mangat PK, Garrett-Mayer E, Halabi S, Hansra D, Calfa CJ, Khalil MF, Ahn ER, Cannon TL, Crilley P et al (2021) Pembrolizumab in patients with metastatic breast cancer with high tumor mutational burden: results from the targeted agent and profiling utilization registry (TAPUR) study. J Clin Oncol 39(22):2443–2451
Ijaz A, Khan AY, Malik SU, Faridi W, Fraz MA, Usman M, Tariq MJ, Durer S, Durer C, Russ A et al (2019) Significant risk of graft-versus-host disease with exposure to checkpoint inhibitors before and after allogeneic transplantation. Biol Blood Marrow Transplant 25(1):94–99
Soiffer RJ, Davids MS, Chen YB (2018) Tyrosine kinase inhibitors and immune checkpoint blockade in allogeneic hematopoietic cell transplantation. Blood 131(10):1073–1080
Acknowledgements
The authors wish to acknowledge Chris Davis for his help in accessing transplantation data for this patient population.
Funding
This work was supported by NIH/NCI, P30 CA015704, Wendy Leisenring, P30 CA015704, Hannah Linden.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Doney, K., Leisenring, W. & Linden, H. Allogeneic hematopoietic cell transplantation in patients with a hematologic malignancy and a prior history of breast cancer. Breast Cancer Res Treat 194, 507–516 (2022). https://doi.org/10.1007/s10549-022-06658-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06658-5