Abstract
Purpose
Metastasis is the main cause of breast cancer mortality. Recent studies have proved that lipid metabolic reprogramming plays critical roles in breast cancer carcinogenesis and metastasis. We aim to identify critical lipid metabolism genes in breast cancer metastasis.
Methods
We designed and cloned a CRISPR pooled library containing lipid metabolic gene guide RNAs and performed a genetic screen in vivo. Transwell assay and animal experiments were used to evaluate cell metastatic ability in vitro or in vivo, respectively. We performed immunohistochemistry with breast cancer tissue microarray to study the clinical significance of NSDHL.
Findings
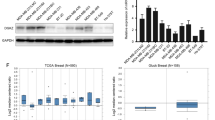
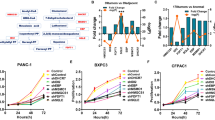
We identified a cholesterol metabolic enzyme, NSDHL, as a potential metastatic driver in triple-negative breast cancer. NSDHL was highly expressed in breast cancer tissues and predicted a poor prognosis. NSDHL knockdown significantly suppressed cell proliferation and migration. Mechanistically, NSDHL activated the TGFβ signaling pathway by inhibiting the endosomal degradation of TGFβR2. In addition, blocking the upstream metabolism of NSDHL with ketoconazole rescued cancer metastasis and TGFβR2 degradation. However, the inactivation of NSDHL (Y151X) did not rescue the migration ability and the TGFβR2 protein expression.
Conclusion
Taken together, our findings established that NSDHL serves as a metastatic driver, and its function depends on its enzyme activity in cholesterol biosynthesis and is mediated by the NSDHL-TGFβR2 signal pathway. Our study indicated that NSDHL and steroid biosynthesis may serve as new drug targets for patients with advanced breast cancer.





Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal J (2018) Cancer statistics. CA 68(1):7–30. https://doi.org/10.3322/caac.21442
Eng LG, Dawood S, Sopik V, Haaland B, Tan PS, Bhoo-Pathy N, Warner E, Iqbal J, Narod SA, Dent R (2016) Ten-year survival in women with primary stage IV breast cancer. Breast Cancer Res Treat 160(1):145–152. https://doi.org/10.1007/s10549-016-3974-x
Dibaba DT, Ogunsina K, Braithwaite D, Akinyemiju T (2018) Metabolic syndrome and risk of breast cancer mortality by menopause, obesity, and subtype. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-018-5056-8
Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM (2017) Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA 67(5):378–397. https://doi.org/10.3322/caac.21405
Park J, Morley TS, Kim M, Clegg DJ, Scherer PE (2014) Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol 10(8):455–465. https://doi.org/10.1038/nrendo.2014.94
Menendez JA, Lupu R (2017) Fatty acid synthase (FASN) as a therapeutic target in breast cancer. Expert Opin Ther Targets 21(11):1001–1016. https://doi.org/10.1080/14728222.2017.1381087
Patel SJ, Sanjana NE, Kishton RJ, Eidizadeh A, Vodnala SK, Cam M, Gartner JJ, Jia L, Steinberg SM, Yamamoto TN, Merchant AS, Mehta GU, Chichura A, Shalem O, Tran E, Eil R, Sukumar M, Guijarro EP, Day CP, Robbins P, Feldman S, Merlino G, Zhang F, Restifo NP (2017) Identification of essential genes for cancer immunotherapy. Nature 548(7669):537–542. https://doi.org/10.1038/nature23477
Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F (2014) Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343(6166):84–87. https://doi.org/10.1126/science.1247005
Chen S, Sanjana NE, Zheng K, Shalem O, Lee K, Shi X, Scott DA, Song J, Pan JQ, Weissleder R, Lee H, Zhang F, Sharp PA (2015) Genome-wide CRISPR screen in a mouse model of tumor growth and metastasis. Cell 160(6):1246–1260. https://doi.org/10.1016/j.cell.2015.02.038
Bergqvist C, Abdallah B, Hasbani DJ, Abbas O, Kibbi AG, Hamie L, Kurban M (2018) CHILD syndrome: a modified pathogenesis-targeted therapeutic approach. Am J Med Genet A 176(3):733–738. https://doi.org/10.1002/ajmg.a.38619
Sukhanova A, Gorin A, Serebriiskii IG, Gabitova L, Zheng H, Restifo D, Egleston BL, Cunningham D, Bagnyukova T, Liu H, Nikonova A, Adams GP, Zhou Y, Yang DH, Mehra R, Burtness B, Cai KQ, Klein-Szanto A, Kratz LE, Kelley RI, Weiner LM, Herman GE, Golemis EA, Astsaturov I (2013) Targeting C4-demethylating genes in the cholesterol pathway sensitizes cancer cells to EGF receptor inhibitors via increased EGF receptor degradation. Cancer Discov 3(1):96–111. https://doi.org/10.1158/2159-8290.cd-12-0031
Zheng YZ, Xue MZ, Shen HJ, Li XG, Ma D, Gong Y, Liu YR, Qiao F, Xie HY, Lian B, Sun WL, Zhao HY (2018) PHF5A epigenetically inhibits apoptosis to promote breast cancer progression. Can Res 78(12):3190–3206. https://doi.org/10.1158/0008-5472.can-17-3514
Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R (2001) A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res 16(8):1486–1495. https://doi.org/10.1359/jbmr.2001.16.8.1486
Wu FT, Lee CR, Bogdanovic E, Prodeus A, Gariépy J, Kerbel RS (2015) Vasculotide reduces endothelial permeability and tumor cell extravasation in the absence of binding to or agonistic activation of Tie2. EMBO Mol Med 7(6):770–787. https://doi.org/10.15252/emmm.201404193
Li W, Xu H, Xiao T, Cong L, Love MI, Zhang F, Irizarry RA, Liu JS, Brown M, Liu XS (2014) MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol 15(12):554. https://doi.org/10.1186/s13059-014-0554-4
Sun HF, Yang XL, Zhao Y, Tian Q, Chen MT, Zhao YY, Jin W (2019) Loss of TMEM126A promotes extracellular matrix remodeling, epithelial-to-mesenchymal transition, and breast cancer metastasis by regulating mitochondrial retrograde signaling. Cancer Lett 440–441:189–201. https://doi.org/10.1016/j.canlet.2018.10.018
Kavanagh KL, Jornvall H, Persson B, Oppermann U (2008) Medium- and short-chain dehydrogenase/reductase gene and protein families: the SDR superfamily: functional and structural diversity within a family of metabolic and regulatory enzymes. Cell Mol Life Sci 65(24):3895–3906. https://doi.org/10.1007/s00018-008-8588-y
Chen MT, Sun HF, Zhao Y, Fu WY, Yang LP, Gao SP, Li LD, Jiang HL, Jin W (2017) Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population-based analysis. Sci Rep 7(1):9254. https://doi.org/10.1038/s41598-017-10166-8
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J (2005) Genes that mediate breast cancer metastasis to lung. Nature 436(7050):518–524. https://doi.org/10.1038/nature03799
Vishal M, Swetha R, Thejaswini G, Arumugam B, Selvamurugan N (2017) Role of Runx2 in breast cancer-mediated bone metastasis. Int J Biol Macromol 99:608–614. https://doi.org/10.1016/j.ijbiomac.2017.03.021
Gueguinou M, Crottes D, Chantome A, Rapetti-Mauss R, Potier-Cartereau M, Clarysse L, Girault A, Fourbon Y, Jezequel P, Guerin-Charbonnel C, Fromont G, Martin P, Pellissier B, Schiappa R, Chamorey E, Mignen O, Uguen A, Borgese F, Vandier C, Soriani O (2017) The SigmaR1 chaperone drives breast and colorectal cancer cell migration by tuning SK3-dependent Ca(2+) homeostasis. Oncogene 36(25):3640–3647. https://doi.org/10.1038/onc.2016.501
Pascual G, Avgustinova A, Mejetta S, Martin M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A, Hueto JA, Bescos C, Di Croce L, Benitah SA (2017) Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541(7635):41–45. https://doi.org/10.1038/nature20791
Chen L, Yang L, Yao L, Kuang XY, Zuo WJ, Li S, Qiao F, Liu YR, Cao ZG, Zhou SL, Zhou XY, Yang WT, Shi JX, Huang W, Hu X, Shao ZM (2018) Characterization of PIK3CA and PIK3R1 somatic mutations in Chinese breast cancer patients. Nat Commun 9(1):1357. https://doi.org/10.1038/s41467-018-03867-9
Caldas H, Herman GE (2003) NSDHL, an enzyme involved in cholesterol biosynthesis, traffics through the Golgi and accumulates on ER membranes and on the surface of lipid droplets. Hum Mol Genet 12(22):2981–2991. https://doi.org/10.1093/hmg/ddg321
Xue T, Zhang Y, Zhang L, Yao L, Hu X, Xu LX (2013) Proteomic analysis of two metabolic proteins with potential to translocate to plasma membrane associated with tumor metastasis development and drug targets. J Proteome Res 12(4):1754–1763. https://doi.org/10.1021/pr301100r
Gabitova L, Restifo D, Gorin A, Manocha K, Handorf E, Yang DH, Cai KQ, Klein-Szanto AJ, Cunningham D, Kratz LE, Herman GE, Golemis EA, Astsaturov I (2015) Endogenous Sterol Metabolites Regulate Growth of EGFR/KRAS-Dependent Tumors via LXR. Cell Rep 12(11):1927–1938. https://doi.org/10.1016/j.celrep.2015.08.023
Beck TN, Georgopoulos R, Shagisultanova EI, Sarcu D, Handorf EA, Dubyk C, Lango MN, Ridge JA, Astsaturov I, Serebriiskii IG, Burtness BA, Mehra R, Golemis EA (2016) EGFR and RB1 as dual biomarkers in HPV-negative head and neck cancer. Mol Cancer Ther 15(10):2486–2497. https://doi.org/10.1158/1535-7163.mct-16-0243
Yoon SH, Kim HS, Kim RN, Jung SY, Hong BS, Kang EJ, Lee HB, Moon HG, Noh DY, Han W (2020) NAD(P)-dependent steroid dehydrogenase-like is involved in breast cancer cell growth and metastasis. BMC Cancer 20(1):375. https://doi.org/10.1186/s12885-020-06840-2
Ikushima H, Miyazono K (2010) TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer 10(6):415–424. https://doi.org/10.1038/nrc2853
Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E (2009) Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol 11(11):1287–1296. https://doi.org/10.1038/ncb1973
Baek AE, Yu YA, He S, Wardell SE, Chang CY, Kwon S, Pillai RV, McDowell HB, Thompson JW, Dubois LG, Sullivan PM, Kemper JK, Gunn MD (2017) The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat Commun 8(1):864. https://doi.org/10.1038/s41467-017-00910-z
Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, Umetani M, Geradts J, McDonnell DP (2013) 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 342(6162):1094–1098. https://doi.org/10.1126/science.1241908
Kim DG, Cho S, Lee KY, Cheon SH, Yoon HJ, Lee JY, Kim D, Shin KS, Koh CH, Koo JS, Choi Y, Lee HH, Oh YK, Jeong YS, Chung SJ, Baek M, Jung KY, Lim HJ, Kim HS, Park SJ, Lee JY, Lee SJ, Lee BJ (2021) Crystal structures of human NSDHL and development of its novel inhibitor with the potential to suppress EGFR activity. Cell Mol Life Sci 78(1):207–225. https://doi.org/10.1007/s00018-020-03490-2
Shehu A, Albarracin C, Devi YS, Luther K, Halperin J, Le J, Mao J, Duan RW, Frasor J, Gibori G (2011) The stimulation of HSD17B7 expression by estradiol provides a powerful feed-forward mechanism for estradiol biosynthesis in breast cancer cells. Mol Endocrinol 25(5):754–766. https://doi.org/10.1210/me.2010-0261
Haynes BP, Straume AH, Geisler J, A’Hern R, Helle H, Smith IE, Lonning PE, Dowsett M (2010) Intratumoral estrogen disposition in breast cancer. Clin Cancer Res 16(6):1790–1801. https://doi.org/10.1158/1078-0432.ccr-09-2481
Langballe R, Cronin-Fenton D, Dehlendorff C, Jensen MB, Ejlertsen B, Andersson M, Friis S, Mellemkjaer L (2018) Statin use and risk of contralateral breast cancer: a nationwide cohort study. Br J Cancer 119(10):1297–1305. https://doi.org/10.1038/s41416-018-0252-1
Borgquist S, Giobbie-Hurder A, Ahern TP, Garber JE, Colleoni M, Lang I, Debled M, Ejlertsen B, von Moos R, Smith I, Coates AS, Goldhirsch A, Rabaglio M, Price KN, Gelber RD, Regan MM, Thurlimann B (2017) Cholesterol, cholesterol-lowering medication use, and breast cancer outcome in the BIG 1–98 study. J Clin Oncol 35(11):1179–1188. https://doi.org/10.1200/jco.2016.70.3116
Funding
This work was supported by the grant from National Natural Science Foundation of China (Grant No.: 81972727).
Author information
Authors and Affiliations
Contributions
Conception and design: MC, HS, WJ. Development of methodology: HS. Acquisition of data: MC, YZ, YZ, QL. Analysis and interpretation of data: YL, HS. Writing, review, and/or revision of the manuscript: MC, HS, YH, WJ. Administrative, technical, or material support: XY. Study supervision: WJ.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, M., Zhao, Y., Yang, X. et al. NSDHL promotes triple-negative breast cancer metastasis through the TGFβ signaling pathway and cholesterol biosynthesis. Breast Cancer Res Treat 187, 349–362 (2021). https://doi.org/10.1007/s10549-021-06213-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06213-8