Abstract
Purpose
To compare the diagnostic performance of ring-type dedicated breast PET (dbPET), whole-body PET (WBPET), and DCE-MRI for predicting pathological complete response (pCR) after neoadjuvant chemotherapy (NAC).
Methods
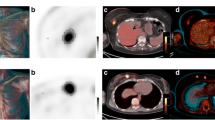
This prospective study included 29 women with histologically proven breast cancer on needle biopsy between July 2016 and July 2019 (age: mean 55 years; range 35–78). Patients underwent WBPET followed by ring-type dbPET and DCE-MRI pre- and post-NAC for preoperative evaluation. pCR was defined as an invasive tumor that disappeared in the breast. Standardized uptake values corrected for lean body mass (SULpeak) were calculated for dbPET and WBPET scans. Maximum tumor length was measured in DCE-MRI images.
Reduction rates were calculated for quantitative evaluation. Two radiologists independently evaluated the qualitative findings. Reduction rates and qualitative findings were compared between the pCR (n = 7) and non-pCR (n = 22) groups for each modality. Differences in quantitative and qualitative data between the two groups were analyzed statistically.
Results
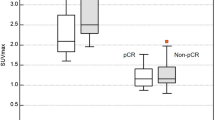
Significant differences were observed in the reduction rates of dbPET and DCE-MRI (P = 0.01 and 0.03, respectively) between the two groups. Univariate and multiple logistic regression analyses revealed that SULpeak reduction rates in WBPET and dbPET (P = 0.02 and P = 0.01, respectively) and in dbPET (odds ratio, 16.00; 95% CI 1.57–162.10; P = 0.01) were significant indicators associated with pCR, respectively. No between-group differences were observed in qualitative findings in the three modalities.
Conclusion
SULpeak reduction rate of dbPET > 82% was an independent indicator associated with pCR after NAC in breast cancer.



Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics. CA Cancer J Clin 70:7–30. https://doi.org/10.3322/caac.21590
Rastogi P, Anderson SJ, Bear HD et al (2008) Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol 26:778ee85. https://doi.org/10.1200/JCO.2007.15.0235
von Minckwitz G, Untch M, Blohmer JU et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804. https://doi.org/10.1200/JCO.2011.38.8595
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172. https://doi.org/10.1016/S0140-6736(13)62422-8
Yuan Y, Chen XS, Liu SY, Shen KW (2010) Accuracy of MRI in prediction of pathologic complete remission in breast cancer after preoperative therapy: a metaanalysis. AJR AmJ Roentrenol 195:260–268. https://doi.org/10.2214/AJR.09.3908
Hylton NM, Blume JD, Bernreuter WK et al (2012) Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy—results from ACRIN 6657/I-SPY TRIAL. Radiology 263:663–672. https://doi.org/10.1148/radiol.12110748
Negrão EMS, Bitencourt AGV, de Souza JA, Marques EF (2019) Accuracy of breast magnetic resonance imaging in evaluating the response to neoadjuvant chemotherapy: a study of 310 cases at a cancer center. Radiol Bras 52:299–304. https://doi.org/10.1590/0100-3984.2018.0149
Fowler AM (2014) A molecular approach to breast imaging. J Nucl Med 55:177e80. https://doi.org/10.2967/jnumed.113.126102
Boellaard R (2009) Standards for PET image acquisition and quantitative data analysis. J Nucl Med 50(Suppl 1):11S-20S. https://doi.org/10.2967/jnumed.108.057182
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Tateishi U, Miyake M, Nagaoka T et al (2012) Neoadjuvant chemotherapy in breast cancer: prediction of pathologic response with PET/CT and dynamic contrast-enhanced MR imaging–prospective assessment. Radiology 263:53–63. https://doi.org/10.1148/radiol.12111177
Riedl CC, Pinker K, Ulaner GA et al (2017) Comparison of FDG-PET/CT and contrast-enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur J Nucl Med Mol Imaging 44:1428–1437. https://doi.org/10.1007/s00259-017-3703-7
Kitajima K, Miyoshi Y, Yamano T, Odawara S, Higuchi T, Yamakado K (2018) Assessment of tumor response to neoadjuvant chemotherapy in patients with breast cancer using MRI and FDG-PET/CT-RECIST 1.1 vs. PERCIST 1.0. Nagoya J Med Sci 80:183–197. https://doi.org/10.18999/nagjms.80.2.183
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S-150S. https://doi.org/10.2967/jnumed.108.057307
D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA (2013) ACR BI-RADS Atlas, breast imaging reporting and data system, 5th edn. American College of Radiology, Reston, VA
García Hernández T, Vicedo González A, Ferrer Rebolleda J et al (2016) Performance evaluation of a high resolution dedicated breast PET scanner. Med Phys 43:2261. https://doi.org/10.1118/1.4945271
Nishimatsu K, Nakamoto Y, Miyake KK et al (2017) Higher breast cancer conspicuity on dbPET compared to WB-PET/CT. Eur J Radiol 90:138–145. https://doi.org/10.1016/j.ejrad.2017.02.046
Satoh Y, Motosugi U, Imai M, Onishi H (2020) Comparison of dedicated breast positron emission tomography and whole-body positron emission tomography/computed tomography images: a common phantom study. Ann Nucl Med 34:119–127. https://doi.org/10.1007/s12149-019-01422-0
Caprio MG, Cangiano A, Imbriaco M et al (2010) Dual-time-point [18F]-FDG PET/CT in the diagnostic evaluation of suspicious breast lesions. La Radiol Med 115:215–224
Kim HO, Kim BS, Kang SY et al (2020) Metabolic changes in breast cancer on dual-time-point 18F-FDG PET/CT imaging according to primary tumor uptake and background parenchymal enhancement. Ann Nucl Med 34:942–951. https://doi.org/10.1007/s12149-020-01525-z
Sasada S, Masumoto N, Goda N et al (2018) Dedicated breast PET for detecting residual disease after neoadjuvant chemotherapy in operable breast cancer: a prospective cohort study. Eur J Surg Oncol 44:444–448. https://doi.org/10.1016/j.ejso.2018.01.014
Koyasu H, Goshima S, Noda Y et al (2019) The feasibility of dedicated breast PET for the assessment of residual tumor after neoadjuvant chemotherapy. Jpn J Radiol 37:81–87. https://doi.org/10.1007/s11604-018-0785-5
Rauch GM, Adrada BE, Kuerer HM, van la Parra RF, Leung JW, Yang WT (2017) Multimodality imaging for evaluating response to neoadjuvant chemotherapy in breast cancer. AJR Am J Roentgenol 208:290–299. https://doi.org/10.2214/AJR.16.17223
Dose-Schwarz J, Tiling R, Avril-Sassen S et al (2010) Assessment of residual tumour by FDG-PET: conventional imaging and clinical examination following primary chemotherapy of large and locally advanced breast cancer. Br J Cancer 102:35–41. https://doi.org/10.1038/sj.bjc.6605427
Abdel Razek AA, Gaballa G, Denewer A, Tawakol I (2010) Diffusion weighted MR imaging of the breast. Acad Radiol 17:382–386. https://doi.org/10.1016/j.acra.2009.10.014
Abdel Razek AAK, Zaky M, Bayoumi D, Taman S, Abdelwahab K, Alghandour R (2019) Diffusion tensor imaging parameters in differentiation recurrent breast cancer from post-operative changes in patients with breast-conserving surgery. Eur J Radiol 111:76–80. https://doi.org/10.1016/j.ejrad.2018.12.022
Razek AA, Lattif MA, Denewer A, Farouk O, Nada N (2016) Assessment of axillary lymph nodes in patients with breast cancer with diffusion-weighted MR imaging in combination with routine and dynamic contrast MR imaging. Breast Cancer 23:525–532. https://doi.org/10.1007/s12282-015-0598-7
Grimm LJ, Mazurowski MA (2020) Breast cancer radiogenomics: current status and future directions. Acad Radiol 27:39–46. https://doi.org/10.1016/j.acra.2019.09.012
Le EPV, Wang Y, Huang Y, Hickman S, Gilbert FJ (2019) Artificial intelligence in breast imaging. Clin Radiol 74:357–366. https://doi.org/10.1016/j.crad.2019.02.006
Acknowledgements
The authors declare no conflicts of interest associated with this manuscript. We thank Mamoru Furuyashiki, RT, Akira Kida, RT, Risa Momoi, RT, and Shima Inoue, RT, for examination of patients with WBPET and dbPET in the MI Clinic and for support with the measurement of quantitative data. We would like to thank Editage (www.editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest associated with this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tokuda, Y., Yanagawa, M., Fujita, Y. et al. Prediction of pathological complete response after neoadjuvant chemotherapy in breast cancer: comparison of diagnostic performances of dedicated breast PET, whole-body PET, and dynamic contrast-enhanced MRI. Breast Cancer Res Treat 188, 107–115 (2021). https://doi.org/10.1007/s10549-021-06179-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06179-7