Abstract
Background
KN motif and ankyrin repeat domains 1 (KANK1) plays an important role in cytoskeleton maintenance and contributes to the regulation of cell proliferation, adhesion and apoptosis. KANK1 is involved in progression of a variety of solid tumours; however, its role in invasive breast cancer (BC) remains unknown. This study aims to evaluate the clinicopathological and prognostic value of KANK1 expression in operable BC.
Methods
KANK1 expression was assessed at the transcriptomic level using multiple BC cohorts; the Molecular Taxonomy of BC International Consortium cohort (METABRIC; n = 1980), The Cancer Genome Atlas BC cohort (TCGA; n = 949) and the publicly available BC transcriptomic data hosted by BC Gene-Expression Miner (bc-GenExMiner v4.0) and Kaplan–Meier plotter?. The Nottingham BC cohort (n = 1500) prepared as tissue microarrays was used to assess KANK1 protein expression using immunohistochemistry (IHC). The association between clinicopathological variables and patient outcome was investigated.
Results
In the METABRIC cohort, high expression of KANK1 mRNA was associated with characteristics of good prognosis including lower grade, absence of lymphovascular invasion and HER2 negativity (all; p < 0.001) and with better outcome [p = 0.006, Hazards ratio, (HR) 0.70, 95% CI 0.54–0.91]. High KANK1 protein expression was correlated with smaller tumour size and HER2 negativity, and better outcome in terms of longer breast cancer-specific survival [p = 0.013, HR 0.7, 95% CI 0.536–0.893] and time to distant metastasis [p = 0.033, HR 0.65, 95% CI 0.51–0.819].
Conclusion
These results supported that upregulation of KANK1 works as a tumour suppressor gene in BC and is associated with improved patients’ outcomes.
Similar content being viewed by others
Background
Breast cancer (BC) is a heterogeneous disease associated with a variety of morphological, molecular features, outcomes and response to therapy [1]. Although BC outcome has improved over the years, 20–30% of patients develop distant metastasis with subsequent poor outcome [2]. Several mechanisms are involved in BC metastasis; however, the key molecular factors driving metastasis remain to be defined.
KN motif and ankyrin repeat domains 1 gene (KANK1) is located at chromosome 9p24 [3] and is composed of KANK N-terminal (KN) motif, the central coiled-coil domains and the C-terminal ankyrin (ANK) repeats [4]. Notably, KANK1 protein interacts with other associated proteins via the coiled-coil and the ankyrin repeat domains, respectively [4]. KANK1 has an essential role in cytoskeleton maintenance via regulating the rate of cytoskeleton proteins production and controlling actin polymerisation [4]. KANK1 plays an important role in the down-regulation of the Rho-associated kinase (ROCK) pathway [5], which is recognised to be involved in various cellular functions such as proliferation, adhesion, cell differentiations and apoptosis [6]. This allow KANK1 to integrate alongside with β-catenin aiming to regulate its distribution in the nucleus and concentrate its transcription, therefore, affecting the development of cancer [7]. Importantly, several in vivo studies revealed a link between the ROCK pathway and tumour cell metastasis [8, 9] and indicated its role in multiple human cancers including BC [6].
The signalling processes controlled by KANK1 expression are also involved in the regulation of epithelial mesenchymal transmission (EMT) by cooperating with transforming growth factor-β (TGF-β) to induce the cytoskeletal reorganisation [10]. KANK1 plays an important role in the development of many malignant tumours. For instance, in vivo KANK1 overexpression reduces the tumorigenicity in lung cancer [11]. Further, in vivo and in vitro studies confirm that KANK1 upregulation in gastric cancer leads to a decrease in the metastatic ability of tumour cells [12]. However, the prognostic significance of KANK1 expression in BC remains unclear. This study aimed to assess the biological and clinical significance of KANK1 mRNA and KANK1 protein expression in BC and the association between KANK1 mRNA expressions with EMT-related genes.
Materials and methods
Study cohorts
KANK1 transcriptomic data
The molecular taxonomy of breast cancer international consortium (METABRIC) dataset (n = 1980) [13] was used to evaluate KANK1 mRNA expression. In the METABRIC, mRNA extracted from primary tumour samples was assayed using the Illumina Human HT-12 v3 platforms (Illumina, Inc., San Diego, USA). Gene-expression data were prepared and normalised as described previously [14]. Furthermore, The Cancer Genome Atlas (TCGA) BC dataset (n = 895) [15] was used to evaluate KANK1 mRNA expression. In the TCGA cohort, RNASeqV2 data and clinicopathological information provided by cBioPortal were used [16, 17]. The prognostic value of KANK1 mRNA expression was further evaluated using the online Breast Cancer Gene-Expression Miner v4.0 (bc-GenExMiner v4.0) database (n = 3871) [18] and the Kaplan–Meier plotter (n = 1402) [19].
KANK1 protein expression
KANK1 protein cohort
A well-characterised cohort of primary operable BC was incorporated in this study, in which the cases were collected from patients presented to Nottingham City Hospital, NHS Trust between 1998 and 2006 (Supplementary Table 1). The Nottingham Prognostic Index (NPI) and oestrogen receptor (ER) status were used to classify patients into clinically relevant groups for management purposes. Based on the NPI, patients were sub-classified into two groups; patients with NPI > 3.4 received tamoxifen if ER status was positive and chemotherapy if ER was negative; however, patients who had NPI ≤ 3.4 received no adjuvant therapy. Patients lacking ER expression (ER) and eligible to receive chemotherapy were treated with classical cyclophosphamide, methotrexate and 5-flurouacil (CMF). Neoadjuvant therapy or anti-Her2-targeted therapy was not used to treat patients in this study. Information of therapy, clinical history and outcomes are prospectively maintained. Outcome data included development and time to distant metastasis (TTDM) and breast cancer-specific survival (BCSS) [20]. BCSS was defined as the duration (in months) from the date of primary surgery to the time of death because of BC. Distant metastasis-free interval was defined as the duration (in months) from primary surgical treatment to the occurrence of first distant recurrence. The distribution of clinicopathological parameters between the discovery cohort (METABRIC) and the validation cohort (Nottingham) presented no statistical differences (all correlation coefficients (r) = 0.80, all p < 0.001).
Immunohistochemistry (IHC)
KANK1 antibody (rabbit polyclonal SAB500862; SIGMA Company, USA) specificity was assessed by western blot using human BC cell lysates from MCF7 and SKBR3 (obtained from the American Type culture Collection; Rockville, MD, USA). KANK1 antibody (1:500 dilution) was incubated overnight and showed a single band at the expected molecular weight – 90 kDa and mouse β-actin (A5441, Sigma-Aldrich; Clone AC-15; Sigma, UK) at 1:5000 was used as a house-keeping protein (Fig. 1a).
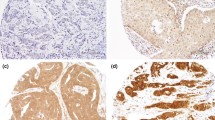
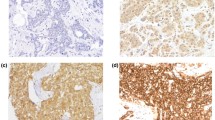
Western blot and immunohistochemical expression of KANK1 in BC. a Western blotting results for KANK1 expression in MCF7 and SKBR3 breast cancer cell lines using rabbit polyclonal antibody against human KANK1 (details). Green bands represent KANK1. Morphological characteristics of KANK1 immunohistochemistry in full-face breast cancer tissue. b–d Normal mammary gland cells showed uniformly strong KANK1 staining. b The reactivity of myoepithelial cells (c) was lower than those of epithelial cells (white arrow: normal epithelial cells). Invasive cancer cells (d) showed uniformly weak KANK1 staining. The reactivity was mainly observed in the cytoplasm. KANK1 protein expression in breast cancer TMA cores (e, f). Showing weak staining (e, f) strong staining in the cytoplasm of cancer cells
To evaluate the pattern and distribution of KANK1 protein expression, full-face tissue sections (n = 14), representative of different BC molecular subtypes and tumour grades, were stained. Tumour samples were arrayed onto TMAs as previously described using the TMA Grand Master® (3D HISTECH®, Budapest, Hungary) [21]. The Novolink Max Polymer Detection system (Leica, Newcastle, UK) was used to detect the immunoreactivity of KANK1. Heat-induced retrieval of antigen epitopes was performed in citrate solution (pH 6.0). KANK1 antibody was incubated at room temperature for 1 h (dilution 1:1500).
Scoring of KANK1 protein expression
The stained slides were scanned into high-resolution digital images at ×20 magnification using a Nanozoomer scanner (Hamamatsu Photonics, Welwyn Garden City, UK). KANK1 cytoplasmic immunoreactivity was evaluated using the modified H-score taking the staining intensity and percentage of positivity into account. Staining intensity (0–3) was multiplied by the proportion of tumour cells (0–100) stained with each intensity and final scores were obtained, giving a range of 0–300 [22]. Double scoring was assessed blindly by two researchers to evaluate the inter-observer concordance. Intraclass correlation coefficient (ICC) concordance between both observers was 0.9.
Statistical analysis
SPSS (IBM SPSS Statistic, Version 24.0) was used in statistical analysis. Pearson correlation test was used to evaluate the relationship of KANK1 mRNA expression with the expression of a set of genes known to be associated with EMT and cancer cell migration (CDH1, CDH2, TGFB, TWIST2, TWIST1, ZEB2, ZEB1 SLUG, SNAIL, NFKB1, LLGL2, GSK3B, CRUMBS and CTNNB1). The correlation between KANK1 expression and clinicopathological factors was analysed using Chi-square test. Kaplan–Meier survival curves using the log-rank test were used to assess the prognostic significance of KANK1 expression. Cox proportional hazard method was employed for the multivariate survival analysis. KANK1 mRNA/protein expression did not follow a normal distribution and was dichotomised using median cut-off values (95). The p value < 0.05 (two-tailed) was considered statistically significant for clinicopathological parameters and survival. This work was preformed according to REMARK guidelines or tumour prognostic study [23], and approved ethically approval by the North West–Greater Manchester Central Research Ethics Committee under the title: Nottingham Health Science Biobank (NHSB), reference number 15/NW/0685.
Results
Clinicopathological significance of KANK1 mRNA expression
High KANK1 mRNA expression was significantly indicative of good prognosis as cases with high KANK1 mRNA expression had better BCSS outcome compared to low KANK1 mRNA expression (p = 0.036; Fig. 2a). Similar associations were observed in the bc-GenExMiner v4.0 and KM plotter BC datasets (Supplementary Fig. 1a, b). High KANK1 mRNA expression was also associated with improved outcome when restricting the analysis to subgroups including ER negativity (METABRIC: p = 0.007; TCGA: p < 0.001), HER2 negativity (METABRIC: p < 0.001; TCGA: p < 0.001) and LVI negativity (METABRIC: p = 0.005; TCGA: p = 0.003; Table 1).
KANK1 patient overall survival and time to distant metastasis. a METABRIC cohort, BC overall survival was significantly better in high KANK1 mRNA expression group than in the low KANK1 expression group. b KANK1 protein expression BC overall survival was significantly better in the high KANK1 protein expression group than in the low expression group. c KANK1 protein expression BC TTDM was significantly better in the high KANK1 protein expression group than the low expression group
KANK1 mRNA overexpression was associated with higher expression of CDH1 (METABRIC: p = 0.022; TCGA: p < 0.001), CTNNB1 (METABRIC: p < 0.001; TCGA: p < 0.001); however, KANK1 mRNA overexpression was correlated with lower LLGL2 (METABRIC: p = 0.002; TCGA: p < 0.001) (Table 2).
KANK1 protein expression
BC full-face sections showed homogenous cytoplasmic expression of KANK1. KANK1 expression in normal glandular epithelium was uniformly strong (Fig. 1b). KANK1 immunoreactivity of myoepithelial cells was lower than those of glandular epithelial cells (Fig. 1c). In contrast, invasive cancer cells exhibited weaker expression of KANK1 compared to the normal mammary epithelial cells present in some TMA cores (Fig. 1d).
Using the median H-score (95) as a cut-off point, high KANK1 expression was observed in 599/1500 (40%) of tumours (Fig. 1e, f). High KANK1 protein expression was associated with smaller tumour size (p = 0.012) and HER2 positivity (p = 0.007; Table 3).
Those patients with tumours showing high KANK1 protein expression had significantly better 10 years BCSS (p = 0.024; Fig. 2b) and longer TTDM (p = 0.048; Fig. 2c) compared with those patients showing low/reduced KANK1 expression. Multivariate analyses indicated that high KANK1 expression is correlated (< 0.05) with better outcome in terms of longer BCSS and TTDM, independent of other established prognostic variables including tumour size, Nottingham grade, nodal stage, LVI, ER status, PR status and HER2 status (Table 4).
When we stratified our BC cohort based on hormonal receptor and HER2 expression, overexpression of KANK1 protein was predictive of longer BCSS in the receptor-negative subgroups (p = 0.024, p = 0.038 and p = 0.014 for ER−, PR− and HER2− tumours, respectively; Fig. 3a–c). TTDM showed similar association in both ER and HER2-negative BC (p = 0.027 and p = 0.014) (Fig. 3d, e). Importantly, when exploring the value of KANK1 protein expression in TNBC (n = 203), high KANK1 expression was also associated with prolonged survival (BCSS: p = 0.036 and TTDM p = 0.025; Fig. 4).
Molecular BC subtypes overall survival and time to distant metastasis. a ER-negative BC patients’ overall survival. b PR negativity BC patients’ overall survival. c HER2-negative BC patients’ overall survival. d ER-negative BC TTDM patients. e PR-negative BC TTDM patients. f Her2-negative BC TTDM patients
Discussion
This study has robustly demonstrated that high KANK1 expression is associated with good prognostic characteristics and improved BC patients’ outcomes, which is in agreement with other cancers including gastric [11], nerve [24] and lung [25]. Our study also showed that high KANK1 mRNA expression showed improved survival time in the aggressive and clinically relevant subgroups of BC, namely ER, PR and HER2-negative tumours. It was also strongly associated with clinicopathological variables characteristic of good prognosis including LVI negativity and lower grade, highlighting a potential tumour suppressive role in BC.
In the current study, high KANK1 mRNA expression was associated with ER, PR and HER2 negativity. This is consistent with KANK1 protein, except for HER2. This discrepancy in KANK1:HER2 expression between the protein and transcript levels may be attributable to the nature of the cohort, complicated post-transcriptional mechanisms and proteins may differ substantially in their in vivo half-lives [24, 25]. However, due to the relatively small sample size of the HER2-positive subgroup, further confirmation in larger cohorts of both HER2-positive and HER2-negative cases is required to determine the exact role of KANK1 in HER2-positive BC.
Nonetheless, when investigating the role of KANK1 mRNA expression with well-established EMT transcription factors, our data showed a negative correlation between KANK1 mRNA expression and other EMT genes (TGFB1, CDH2, LLGL2 and CTNNB1). On the other hand, high KANK1 mRNA expression showed a significant positive association with E-cadherin gene (CDH1), and these findings suggest that high KANK1 expression is involved in reducing tumour cell migration and influencing the LVI process through reducing the RhoA/ROCK pathway, which has a well-known role in controlling cancer cell migration [7]. TGFB1 acts as an oncogene in tumour progression by inducing cell invasion, dissemination to distant sites and augmenting angiogenesis. CDH2 and LLGL2, which play an important role in EMT activation, were negatively correlated with KANK1 high expression. This suggested that the EMT activation is prohibited by the presence of TGFB1, CDH2 and LLGL2. Chen et al. showed in gastric cancer, increased KANK1 expression was associated with smaller tumour size; results in agreement with our study results in both mRNA and protein levels, implying its role in decreasing cellular proliferation. Similarly, KANK1 may regulate the cell proliferation through inhibiting the phosphorylation of PI3 K/AKT proteins [26]. Smaller tumour size and negative association with TGFB1, CDH2 and LLGL2 strengthen the tumour suppressive role of KANK1.
In the whole BC cohort, high KANK1 protein expression was an independent prognostic marker for improved patients’ outcomes in terms of both BCSS and TTDM. Among subgroups, high expression of KANK1 protein appears to play the most significant survival role in TNBC. As TNBCs are highly resistance to chemotherapy compared to other BC types and strongly associated with worse clinical outcome, our results may indicate the promising role of KANK1 in this aggressive subtype regarding benefit from neoadjuvant chemotherapy and improved overall survival [27].
Our results suggest that loss of expression of KANK1 promotes BC progression. This is in concordance with previous reports indicating that reduced expression of KANK1 facilitates metastasis in different types of cancer and further reinforces its role as a prognostic indicator [11, 24, 25].
In summary, high KANK1 expression in BC is associated with favourable prognostic parameters and is an independent prognostic factor with prolonged patient survival. KANK1 appears to play a role in inhibiting tumour cells proliferation, migration, invasion and metastasis. Further functional studies to decipher the role of KANK1 and its mechanism of action as a tumour suppressive driver of invasive BC is warranted.
Data availability
The authors confirm the data that have been used in this work are available on reasonable request.
References
Bonilla JM, Tabanera MT, Mendoza LR (2017) Breast cancer in the 21st century: from early detection to new therapies. Radiologia 59(5):368–379. https://doi.org/10.1016/j.rx.2017.06.003
Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, Gelmon K (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28(20):3271–3277. https://doi.org/10.1200/jco.2009.25.9820
Cards G (2018) KANK1 Gene (Protein Coding). https://www.genecards.org/cgi-bin/carddisp.pl?gene=KANK1. Accessed 11 July 2018
Kakinuma N, Zhu Y, Wang Y, Roy BC, Kiyama R (2009) Kank proteins: structure, functions and diseases. Cell Mol Life Sci 66(16):2651–2659. https://doi.org/10.1007/s00018-009-0038-y
Rafiq NBM, Nishimura Y, Plotnikov SV, Thiagarajan V, Zhang Z, Shi S, Natarajan M, Viasnoff V, Kanchanawong P, Jones GE, Bershadsky AD (2019) A mechano-signalling network linking microtubules, myosin IIA filaments and integrin-based adhesions. Nat Mater 18(6):638–649. https://doi.org/10.1038/s41563-019-0371-y
Cascione M, De Matteis V, Toma CC, Pellegrino P, Leporatti S, Rinaldi R (2017) Morphomechanical and structural changes induced by ROCK inhibitor in breast cancer cells. Exp Cell Res 360(2):303–309. https://doi.org/10.1016/j.yexcr.2017.09.020
Wang Y, Kakinuma N, Zhu Y, Kiyama R (2006) Nucleo-cytoplasmic shuttling of human Kank protein accompanies intracellular translocation of beta-catenin. J Cell Sci 119(19):4002–4010. https://doi.org/10.1242/jcs.03169
Clark EA, Golub TR, Lander ES, Hynes RO (2000) Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406(6795):532–535. https://doi.org/10.1038/35020106
Murata T, Arii S, Nakamura T, Mori A, Kaido T, Furuyama H, Furumoto K, Nakao T, Isobe N, Imamura M (2001) Inhibitory effect of Y-27632, a ROCK inhibitor, on progression of rat liver fibrosis in association with inactivation of hepatic stellate cells. J Hepatol 35(4):474–481
Korol A, Taiyab A, West-Mays JA (2016) RhoA/ROCK signaling regulates TGFbeta-induced epithelial-mesenchymal transition of lens epithelial cells through MRTF-A. Mol Med 22:713–723. https://doi.org/10.2119/molmed.2016.00041
Weng Z, Shang Y, Yao D, Zhu J, Zhang R (2018) Structural analyses of key features in the KANK1.KIF21A complex yield mechanistic insights into the cross-talk between microtubules and the cell cortex. J Biol Chem 293(1):215–225. https://doi.org/10.1074/jbc.m117.816017
Chen T, Wang K, Tong X (2017) In vivo and in vitro inhibition of human gastric cancer progress by upregulating Kank1 gene. Oncol Rep 38(3):1663–1669. https://doi.org/10.3892/or.2017.5823
Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, Graf S, Ha G, Haffari G, Bashashati A, Russell R, McKinney S, Langerod A, Green A, Provenzano E, Wishart G, Pinder S, Watson P, Markowetz F, Murphy L, Ellis I, Purushotham A, Borresen-Dale AL, Brenton JD, Tavare S, Caldas C, Aparicio S (2012) The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature 486(7403):346–352. https://doi.org/10.1038/nature10983
Craze ML, El-Ansari R, Aleskandarany MA, Cheng KW, Alfarsi L, Masisi B, Diez-Rodriguez M, Nolan CC, Ellis IO, Rakha EA, Green AR (2019) Glutamate dehydrogenase (GLUD1) expression in breast cancer. Breast Cancer Res Treat 174(1):79–91. https://doi.org/10.1007/s10549-018-5060-z
Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, Zhang H, McLellan M, Yau C, Kandoth C, Bowlby R, Shen H, Hayat S, Fieldhouse R, Lester SC, Tse GM, Factor RE, Collins LC, Allison KH, Chen YY, Jensen K, Johnson NB, Oesterreich S, Mills GB, Cherniack AD, Robertson G, Benz C, Sander C, Laird PW, Hoadley KA, King TA, Perou CM (2015) Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163(2):506–519. https://doi.org/10.1016/j.cell.2015.09.033
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2(5):401–404. https://doi.org/10.1158/2159-8290.cd-12-0095
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. https://doi.org/10.1126/scisignal.2004088
Cancer Genome Atlas Network (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418):61–70. https://doi.org/10.1038/nature11412
Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z (2010) An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1809 patients. Breast Cancer Res Treat 123(3):725–731. https://doi.org/10.1007/s10549-009-0674-9
El Ansari R, Craze ML, Miligy I, Diez-Rodriguez M, Nolan CC, Ellis IO, Rakha EA, Green AR (2018) The amino acid transporter SLC7A5 confers a poor prognosis in the highly proliferative breast cancer subtypes and is a key therapeutic target in luminal B tumours. Breast Cancer Res 20(1):21. https://doi.org/10.1186/s13058-018-0946-6
Abd El-Rehim DM, Ball G, Pinder SE, Rakha E, Paish C, Robertson JF, Macmillan D, Blamey RW, Ellis IO (2005) High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J Cancer 116(3):340–350. https://doi.org/10.1002/ijc.21004
McCarty JK, Miller LS, Cox EB, Konrath J, McCarty SK (1985) Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 109(8):716–721
Sauerbrei W, Taube SE, McShane LM, Cavenagh MM, Altman DG (2018) Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): an abridged explanation and elaboration. J Natl Cancer Inst 110(8):803–811. https://doi.org/10.1093/jnci/djy088
Baldi P, Long AD (2001) A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics 17(6):509–519. https://doi.org/10.1093/bioinformatics/17.6.509
Liu Y, Beyer A, Aebersold R (2016) On the dependency of cellular protein levels on mRNA abundance. Cell 165(3):535–550. https://doi.org/10.1016/j.cell.2016.03.014
Kakinuma N, Roy BC, Zhu Y, Wang Y, Kiyama R (2008) Kank regulates RhoA-dependent formation of actin stress fibers and cell migration via 14-3-3 in PI3 K-Akt signaling. J Cell Biol 181(3):537–549. https://doi.org/10.1083/jcb.200707022
Lehmann BD, Jovanovic B, Chen X, Estrada MV, Johnson KN, Shyr Y, Moses HL, Sanders ME, Pietenpol JA (2016) refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS ONE 11(6):e0157368. https://doi.org/10.1371/journal.pone.0157368
Acknowledgments
Yousif Kariri is supported and funded by Shaqra University, Kingdom of Saudi Arabia. We thank Innovate UK for funding (ISCF bid Ref 18181).
Funding
This research was supported and funded by the Saudi Arabia Ministry of Education Shaqra University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Informed consent
This work obtained ethics approval to use the human tissue samples by the North West–Greater Manchester Central Research Ethics Committee under the title: Nottingham Health Science Biobank (NHSB), reference number 15/NW/0685. Informed consent was obtained from all individuals prior to surgery to use their tissue materials in research. This study was performed according to the REMARK guidelines for tumour prognostic studies.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kariri, Y.A., Joseph, C., Kurozumi, S. et al. Prognostic significance of KN motif and ankyrin repeat domains 1 (KANK1) in invasive breast cancer. Breast Cancer Res Treat 179, 349–357 (2020). https://doi.org/10.1007/s10549-019-05466-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05466-8