Abstract
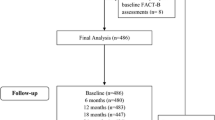
Trials of adjuvant endocrine therapy for breast cancer have shown that aromatase inhibitors have little impact on global health-related quality of life (HRQoL), but have significant effects on patient-reported endocrine symptoms (ESs). There are few studies of HRQoL and psychological distress during preoperative endocrine therapy performed to determine endocrine responsiveness. The NEOS trial is a multicenter, phase 3 randomized controlled trial in postmenopausal women with hormone receptor-positive breast cancer. The primary aim of the trial was to evaluate the need for adjuvant chemotherapy in patients with clinical T1c-T2N0M0, hormone receptor-positive tumors who responded to neoadjuvant letrozole (LET) administered for 24–28 weeks before surgery. The primary endpoint was disease-free survival and the secondary endpoints included adverse events, HRQoL, and cost-effectiveness. In a HRQoL sub-study, subjects were assessed at baseline and 4 and 16 weeks after starting neoadjuvant LET, using the functional assessment of cancer therapy-breast and its ES subscale, and the hospital anxiety and depression scale. HRQoL and psychosocial distress were analyzed in the uncontrolled phase during 24–28 weeks of neoadjuvant LET therapy in the NEOS trial. From May 16, 2008, to December 14, 2011, 503 patients were recruited into the HRQoL sub-study. The full analysis set included 497 patients with a mean age of 63-years old. The questionnaire response rates at enrollment and 4 and 16 weeks were 94.4, 90.7, and 89.1 %, respectively. There were no significant changes in the FACT-G or B-trial outcome index over time, but the social and family well-being score and the ES subscale deteriorated significantly, and the number of patients with clinically significant hot flush increased significantly. Anxiety, depression, and emotional well-being improved significantly after neoadjuvant LET. Neoadjuvant endocrine therapy with LET had no impact on global HRQoL, but did influence endocrine-related symptoms such as hot flush. This study is registered as UMIN000001090.





Similar content being viewed by others
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378:771–784. doi:10.1016/S0140-6736(11)60993-8
Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M, Coombes C, Snowdon C, Gnant M, Jakesz R, Kaufmann M, Boccardo F, Godwin J, Davies C, Peto R (2010) Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 28:509–518. doi:10.1200/JCO.2009.23.1274
Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A (2004) Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) Adjuvant Breast Cancer Trial. J Clin Oncol 22:4261–4271
Cella D, Fallowfield L, Barker P, Cuzick J, Locker G, Howell A (2006) Quality of life of postmenopausal women in the ATAC (“Arimidex”, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for early breast cancer. Breast Cancer Res Treat 100:273–284
Ohsumi S, Shimozuma K, Ohashi Y, Shinji M, Hozumi Y, Mukai H, Takatsuka Y, Aihara T (2011) Health-related quality of life and psychological distress of breast cancer patients after surgery during a phase III randomized trial comparing continuation of tamoxifen with switching to anastrozole after adjuvant tamoxifen for 1-4 years: N-SAS BC 03. Breast Cancer Res Treat 127:143–152. doi:10.1007/s10549-011-1400-y
van Nes JG, Fontein DB, Hille ET, Voskuil DW, van Leeuwen FE, de Haes JC, Putter H, Seynaeve C, Nortier JW, van de Velde CJ (2012) Quality of life in relation to tamoxifen or exemestane treatment in postmenopausal breast cancer patients: a Tamoxifen Exemestane Adjuvant Multinational (TEAM) Trial side study. Breast Cancer Res Treat 134:267–276. doi:10.1007/s10549-012-2028-2
Fallowfield LJ, Bliss JM, Porter LS, Price MH, Snowdon CF, Jones SE, Coombes RC, Hall E (2006) Quality of life in the intergroup exemestane study: a randomized trial of exemestane versus continued tamoxifen after 2–3 years of tamoxifen in postmenopausal women with primary breast cancer. J Clin Oncol 24:910–917
Fallowfield LJ, Kilburn LS, Langridge C, Snowdon CF, Bliss JM, Coombes RC, Trial Steering Committee IES (2012) Long-term assessment of quality of life in the Intergroup Exemestane Study: 5 years post-randomisation. Br J Cancer 106:1062–1067. doi:10.1038/bjc.2012.43
Buijs C, de Vries EG, Mourits MJ, Willemse PH (2008) The influence of endocrine treatments for breast cancer on health-related quality of life. Cancer Treat Rev 34:640–655. doi:10.1016/j.ctrv.2008.04.001
Berry DA, Cirrincione C, Henderson IC, Citron ML, Budman DR, Goldstein LJ, Martino S, Perez EA, Muss HB, Norton L, Hudis C, Winer EP (2006) Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA 295:1658–1667
Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE Jr, Wickerham DL, Wolmark N (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726–3734
Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, Sledge GW, Winer EP, Hudis C, Ingle JN, Perez EA, Pritchard KI, Shepherd L, Gralow JR, Yoshizawa C, Allred DC, Osborne CK, Hayes DF, Breast Cancer Intergroup of North America (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, estrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11:55–65. doi:10.1016/S1470-2045(09)70314-6
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, Wang YC, Bergh J, Di Leo A, Albain K, Swain S, Piccart M, Pritchard K (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379:432–444. doi:10.1016/S0140-6736(11)61625-5
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Shimozuma K, Eguchi N. Development and clinical application of QOL measurement instruments for cancer patients: (I) Japanese versions of QOL questionnaires developed in North American or European countries and validation of reliability and validity of the FACT-B, a QOL questionnaire for breast cancer patients (in Japanese) http://www.jmari.med.or.jp/research/research.php?type=&mode=1&cno=&author=%B2%BC%BA%CA%B9%B8%C6%F3%CF%BA
Shimozuma K, Ohashi Y, Yoshimura K, Iha S, Saeki H, Kuroi K, Katsumata N, Okamoto T, Yamamoto Y, Tanaka K, Sonoo H (2000) Reliability and validity of the Japanese version of the Functional Assessment of Cancer Therapy-Breast (FACT-B) quality-of-life instrument; Women’s Health Outcome Study (WHOS)-01. Qual Life Res 9:287 (abstract 1147)
Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J et al (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11:570–579
Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G (1997) Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol 15:974–986
Fallowfield LJ, Leaity SK, Howell A, Benson S, Cella D (1999) Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat 55:189–199
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67:361–370
Kugaya A, Akechi T, Okuyama T, Okamura H, Uchitomi Y (1998) Screening for psychological distress in Japanese cancer patients. Jpn J Clin Oncol 28:333–338
Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, Buzdar AU, Smith IE, Symmans WF, Singh B, Winer EP (2008) Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol 26:814–819. doi:10.1200/JCO.2007.15.3510
Semiglazov VF, Semiglazov VV, Dashyan GA, Ziltsova EK, Ivanov VG, Bozhok AA, Melnikova OA, Paltuev RM, Kletzel A, Berstein LM (2007) Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer 110:244–254
Shannon C, Smith I (2003) Is there still a role for neoadjuvant therapy in breast cancer? Crit Rev Oncol Hematol 45:77–90
Whelan TJ, Goss PE, Ingle JN, Pater JL, Tu D, Pritchard K, Liu S, Shepherd LE, Palmer M, Robert NJ, Martino S, Muss HB (2005) Assessment of quality of life in MA.17: a randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol 23:6931–6940
Eton DT, Cella D, Yost KJ, Yount SE, Peterman AH, Neuberg DS, Sledge GW, Wood WC (2004) A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol 57:898–910
Stearns V, Ullmer L, López JF, Smith Y, Isaacs C, Hayes D (2002) Hot flushes. Lancet 360:1851–1861
Taira N, Shimozuma K, Shiroiwa T, Ohsumi S, Kuroi K, Saji S, Saito M, Iha S, Watanabe T, Katsumata N (2011) Associations among baseline variables, treatment-related factors and health-related quality of life 2 years after breast cancer surgery. Breast Cancer Res Treat 128:735–747. doi:10.1007/s10549-011-1631-y
Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, Meader N (2011) Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol 12:160–174. doi:10.1016/S1470-2045(11)70002-X
Palmer SC, Taggi A, Demichele A, Coyne JC (2012) Is screening effective in detecting untreated psychiatric disorders among newly diagnosed breast cancer patients? Cancer 118:2735–2743. doi:10.1002/cncr.26603
Bui QU, Ostir GV, Kuo YF, Freeman J, Goodwin JS (2005) Relationship of depression to patient satisfaction: findings from the barriers to breast cancer study. Breast Cancer Res Treat 89:23–28
Shimozuma K, Ganz PA, Petersen L, Hirji K (1999) Quality of life in the first year after breast cancer surgery: rehabilitation needs and patterns of recovery. Breast Cancer Res Treat 56:45–57
Al-Azri M, Al-Awisi H, Al-Moundhri M (2009) Coping with a diagnosis of breast cancer-literature review and implications for developing countries. Breast J 15:615–622. doi:10.1111/j.1524-4741.2009.00812.x
Zainal NZ, Nik-Jaafar NR, Baharudin A, Sabki ZA, Ng CG (2013) Prevalence of depression in breast cancer survivors: a systematic review of observational studies. Asian Pac J Cancer Prev 14:2649–2656
Acknowledgments
This study was sponsored by Public Health Research Foundation, Comprehensive Support Project for Oncology Research (CSPOR). We thank the patients who participated in this trial, and the staff of the CSPOR-Breast Cancer Group for their contributions to this study.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taira, N., Iwata, H., Hasegawa, Y. et al. Health-related quality of life and psychological distress during neoadjuvant endocrine therapy with letrozole to determine endocrine responsiveness in postmenopausal breast cancer. Breast Cancer Res Treat 145, 155–164 (2014). https://doi.org/10.1007/s10549-014-2935-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-2935-5