Abstract
Background
Adjuvant Anastrozole (ANA) for 5 years and Tamoxifen followed by Exemestane (TAM-EXE) for 2.5 years each have become acceptable alternatives to 5 years of Tamoxifen (TAM) for post-menopausal women with breast cancer. As these newer options are associated with higher drug costs as well as improved outcomes, an economic evaluation was undertaken to compare the cost-utility of ANA and TAM-EXE relative to TAM alone and to each other in terms of cost per quality-adjusted life year (QALY) gained.
Methods
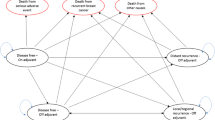
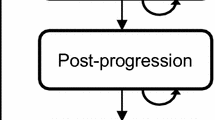
A Markov model was developed to calculate monthly costs and outcomes in a hypothetical cohort of post-menopausal women with early-stage breast cancer. Baseline rates of cancer recurrence and adverse effects with TAM, and hazard ratios associated with ANA and EXE, were derived from the ATAC and IES trials. Patients received hormonal therapy for 5 years and benefit was modeled to persist 5 years beyond treatment. The analysis took a direct payer perspective with a 20-year time horizon. Costs and outcomes were discounted by 3%. Costs are in 2005 Canadian dollars.
Results
ANA and TAM-EXE were associated with increased costs and QALYs, though the cost-utility of both relative to TAM alone was strongly favourable (<$50,000/QALY). Based on an indirect comparison of ANA and TAM-EXE, using TAM alone as a common comparator, the cost-utility of ANA relative to TAM-EXE appears unfavourable.
Conclusions
Both upfront and sequential AI options were cost-effective alternatives to TAM alone, but TAM-EXE appears to be the economically preferred AI option based on its more favourable cost-utility versus ANA.
Similar content being viewed by others
References
Carlson RW, Brown E, Burstein HJ, Gradishar WJ, Hudis CA, Loprinzi C, Mamounas EP, Perez EA, Pritchard K, Ravdin P, Recht A, Somlo G, Theriault RL, Winer EP, Wolff AC, National Comprehensive Cancer Network (2006) NCCN Task Force Report: Adjuvant Therapy for Breast Cancer. J Natl Compr Canc Netw 4(Suppl 1):S1–26
Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, Chlebowski RT, Gelber R, Edge SB, Gralow J, Cobleigh MA, Mamounas EP, Goldstein LJ, Whelan TJ, Powles TJ, Bryant J, Perkins C, Perotti J, Braun S, Langer AS, Browman GP, Somerfield MR (2004) American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol 23(3):619–629
Monnier A (2006) The evolving role of letrozole in the adjuvant setting: first results from the large, phase III, randomized trial BIG 1–98. Breast 15(1 Suppl):21–29
ATAC Trialists Group (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365(9453):60–62
Coombes RC, Hall E, Gibson LJ, et al (2004) A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 350:1081–1091
Coombes RC, Hall E, Snowdon CF, et al (2004) The intergroup exemestane study: a randomized trial in postmenopausal patients with early breast cancer who remain disease-free after two to three years of tamoxifen—updated survival analysis. Breast Cancer Res Treat 88:57(abstr 3)
Ingle JN, Tu D, Pater JL, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Cameron DA, Palmer MJ, Goss PE (2006) Duration of letrozole treatment and outcomes in the placebo-controlled NCIC CTG MA.17 extended adjuvant therapy trial. Breast Cancer Res Treat [Epub ahead of print]
Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardly A, Price KN, Goldhirsch A; Breast International Group (BIG) 1–98 Collaborative Group (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 353(26):2747–2757
Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Cameron DA, Palmer MJ, Pater JL (2005) Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 97(17):1262–1271
Sonnenberg F, Beck R (1993) Markov models in medical decision making: a practical guide. Med Decis Making 13:322–338
Potvin K, Younis T, Sellon M, Barnes P, Rayson D (2005) Patterns of Trastuzumab Use and Cost in a Single Canadian Cancer Institute. Proc ASCO 23(16S):545s, Abstract 6070
Hillner BE (2004) Benefit and projected cost-effectiveness of anastrozole versus tamoxifen as initial adjuvant therapy for patients with early-stage estrogen receptor-positive breast cancer. Cancer 101(6):1311–1322
Early Breast Cancer Trialists’ Collaborative Group (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 351:1451–1467
Electronic Medicines Compendium (2005) Pharmacia, Sandwich, Kent UK http://emc.medicines.org.uk/emc/assets/c/html/displayDocPrinterFriendly.asp?documentid=2484 Cited 23 February 2006
Statistics Canada (2003) Life Tables – Canada, Provinces and Territories, 1995–1997. Cat No. 84–537. Statistics Canada, 2003
Will BP, Berthelot JM, Le Petit C, Tomiak EM, Verma S, Evans WK (2000) Estimates of the lifetime costs of breast cancer treatment in Canada. Eur J Cancer 36(6):724–735
Al-Fozan H, Dufort J, Kaplow M, Valenti D, Tulandi T (2002) Cost analysis of myomectomy, hysterectomy, and uterine artery embolization. Am J Obstet Gynecol 187(5):1401–1404
Lessard C, Framarin A (2003) Endometrial ablation techniques in the treatment of dysfunctional bleeding. Agence d’evaluation technologies et des modes d’intervention en sante, Quebec
Skedgel C, Goeree R, Pleasance S, Thompson K, O’Brien B, Anderson D (2004) The cost-effectiveness of extended antithrombotic prophylaxis following total hip arthroplasty. Value Health 7(6):693, Abstract PCV26
Coyle D, Cranney A, Lee K, Welch V, Tugwell P (2001) Cost effectiveness of nasal calcitonin in postmenopausal women. Pharmacoeconomics 19(5):565–575
Grima D, Burge T, Becker D, Tosteson A (2002) Short-term cost-effectiveness of bisphosphonate therapies for postmenopausal osteoporotic women at high risk of fracture. P&T 27(9):448–455
Consumer Price Index (Health Care) (2005) Statistics Canada, Ottawa, Canada http://dc2.chass.utoronto.ca.ezproxy.library.dal.ca/cgi-bin/cansim2/getSeriesData.pl?s=V1870 2617&b=&e=&f=plain Cited 4 June 2005
Drummond M, O’Brien B, Stoddart G, Torrance G (1997) Methods for the economic evaluation of health care programmes. Oxford Medical publications, Oxford, Great Britain
The CEA Registry (2005) Tufts-New England Medical Center, Institute for Clinical Research and Health Policy Studies http://www.hsph.harvard.edu/cearegistry/data/phaseIIpreferenceweights.pdf Cited 1 June 2005
Punglia R, Kuntz K, Winer E, Weeks J, Burstein H (2005) Optimizing adjuvant endocrine therapy in postmenopausal women with early-stage breast cancer: a decision analysis. J Clin Oncol 32:5178–5187
Buzdar AU, Cuzick J (2006) Anastrozole as an adjuvant endocrine treatment for postmenopausal patients with breast cancer: emerging data. Clin Cancer Res. 12(3 Pt 2):1037s–1048s
Lønning PE (2006) Comparing cost/utility of giving an aromatase inhibitor as monotherapy for 5 years versus sequential administration following 2–3 or 5 years of tamoxifen as adjuvant treatment for postmenopausal breast cancer. Ann Oncol 17(2):217–225
Thompson D, Taylor D, Montoya E, Winer E, Weinstein M (2005) Cost-effectiveness of switching to exemestane following two-to-three years of therapy with tamoxifen in postmenopausal women with primary breast cancer. Breast Cancer Res Treat 94(S1):S218, Abstract 5037
Wilson K, Wordsworth S, Tabberer M, Lees M, Carroll D, Rea D, Coleman R (2006) Exemestane in adjuvant treatment of early breast cancer in postmenopausal women: results of a UK cost-effectiveness model. Eur J Cancer 4(2 Supplement):151, Abstract 354
Lundkvist J, Wilking N, Holmberg S, Lidgren M, Jonsson L (2006) Cost-effectiveness of exemestane versus tamoxifen as adjuvant therapy for early-stage breast cancer after 2–3 years treatment with tamoxifen in Sweden. Eur J Cancer 4(2 Supplement):152, Abstract 358
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Skedgel, C., Rayson, D., Dewar, R. et al. Cost-utility of adjuvant hormone therapies for breast cancer in post-menopausal women: sequential tamoxifen-exemestane and upfront anastrozole. Breast Cancer Res Treat 101, 325–333 (2007). https://doi.org/10.1007/s10549-006-9299-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-006-9299-4