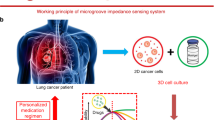
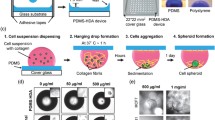
Abstract
To perform the drug screening, planar cultured cell models are commonly applied to test efficacy and toxicity of drugs. However, planar cultured cells are different from the human 3D organs or tissues in vivo. To simulate the human 3D organs or tissues, 3D spheroids are developed by culturing a small aggregate of cells which reside around the extracellular matrix and interact with other cells in liquid media. Here we apply lung carcinoma cell lines to engineer the 3D lung cancer spheroid-based biosensor using the interdigitated electrodes for drug efficacy evaluation. The results show 3D spheroid had higher drug resistance than the planar cell model. The anticarcinogen inhibition on different 3D lung cancer spheroid models (A549, H1299, H460) can be quantitatively evaluated by electric impedance sensing. Besides, we delivered combination of anticarcinogens treatments to A549 spheroids which is commonly used in clinic treatment, and found the synergistic effect of cisplatin plus etoposide had higher drug response. To simultaneously test the drug efficacy and side effects on multi-organ model with circulatory system, a connected multiwell interdigitated electrode arraywas applied to culture different organoid spheroids. Overall, the organization of 3D cancer spheroids-based biosensor, which has higher predictive value for drug discovery and personalized medicine screening, is expected to be well applied in the area of pharmacy and clinical medicine.






Similar content being viewed by others
References
L.R. Arias, C.A. Perry, L. Yang, Real-time electrical impedance detection of cellular activities of oral cancer cells. Biosens. Bioelectron. 25(10), 2225–2231 (2010)
S.S. Árnadóttir, M. Jeppesen, P. Lamy, J.B. Bramsen, I. Nordentoft, M. Knudsen, S. Vang, M.R. Madsen, O. Thastrup, J. Thastrup, Characterization of genetic intratumor heterogeneity in colorectal cancer and matching patient-derived spheroid cultures. Mol. Oncol. 12, 132 (2018)
F. Asphahani, M. Zhang, Cellular impedance biosensors for drug screening and toxin detection. Analyst 132(9), 835–841 (2007)
J. Bierwolf, M. Lutgehetmann, K. Feng, J. Erbes, S. Deichmann, E. Toronyi, C. Stieglitz, B. Nashan, P.X. Ma, J.M. Pollok, Primary rat hepatocyte culture on 3D nanofibrous polymer scaffolds for toxicology and pharmaceutical research. Biotechnol. Bioeng. 108(1), 141–150 (2011)
S.C. Bürgel, L. Diener, O. Frey, J.Y. Kim, A. Hierlemann, Automated, multiplexed electrical impedance spectroscopy platform for continuous monitoring of microtissue spheroids. Anal. Chem. 88(22), 10876–10883 (2016)
E. Fennema, N. Rivron, J. Rouwkema, C. van Blitterswijk, J. de Boer, Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 31(2), 108–115 (2013)
B. Gao, M. Peyton, C. Huang, H. Park, L. Girard, N. Sorrelle, A. Dayoub, R. Brekken, A. Gazdar, J. Minna, 3D spheroid/Organoid models of lung Cancer to study lung Cancer pathogenesis and testing of new therapeutics. J. Thorac. Oncol. 12((8), S1544 (2017)
I. Giaever, C.R. Keese, Use of electric fields to monitor the dynamical aspect of cell behavior in tissue culture. IEEE Trans. Biomed. Eng. (2), 242–247 (1986)
M. Hay, D.W. Thomas, J.L. Craighead, C. Economides, J. Rosenthal, Clinical development success rates for investigational drugs. Nat. Biotechnol. 32(1), 40 (2014)
L. Hu, L. Zou, Z. Qin, J. Fang, L. Huang, P. Wang, A novel label-free bioengineered cell-based biosensor for salicin detection. Sensors Actuators B Chem. 238, 1151–1158 (2017)
G. Huang, J. Pan, Z. Ye, B. Fang, W. Cheng, Z. Cao, Overexpression of miR-216b sensitizes NSCLC cells to cisplatin-induced apoptosis by targeting c-Jun. Oncotarget 8(61), 104206 (2017)
R.W. Jenkins, A.R. Aref, P.H. Lizotte, E. Ivanova, S. Stinson, C.W. Zhou, M. Bowden, J. Deng, H. Liu, D. Miao, Ex vivo profiling of PD-1 blockade using organotypic tumor spheroids. Cancer Discov. 8(2), 196–215 (2018)
C.R. Keese, K. Bhawe, J. Wegener, I. Giaever, Real-time impedance assay to follow the invasive activities of metastatic cells in culture. Biotechniques 33(4), 842–851 (2002)
K.F. Lei, T.K. Liu, N.M. Tsang, Towards a high throughput impedimetric screening of chemosensitivity of cancer cells suspended in hydrogel and cultured in a paper substrate. Biosens. Bioelectron. 100, 355–360 (2018)
J. Li, R. Tao, W. Wu, H. Cao, J. Xin, J. Li, J. Guo, L. Jiang, C. Gao, A.A. Demetriou, 3D PLGA scaffolds improve differentiation and function of bone marrow Mesenchymal stem cell–derived hepatocytes. Stem Cells Dev. 19(9), 1427–1436 (2010)
Y. Li, T. Zhou, C. Ma, W. Song, J. Zhang, Z. Yu, Ginsenoside metabolite compound K enhances the efficacy of cisplatin in lung cancer cells. J. Thorac. Dis. 7(3), 400 (2015)
R.Z. Lin, H.Y. Chang, Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 3(9–10), 1172–1184 (2008)
K. Luongo, A. Holton, A. Kaushik, P. Spence, B. Ng, R. Deschenes, S. Sundaram, S. Bhansali, Microfluidic device for trapping and monitoring three dimensional multicell spheroids using electrical impedance spectroscopy. Biomicrofluidics 7(3), 034108 (2013)
W. Metzger, D. Sossong, A. Bächle, N. Pütz, G. Wennemuth, T. Pohlemann, M. Oberringer, The liquid overlay technique is the key to formation of co-culture spheroids consisting of primary osteoblasts, fibroblasts and endothelial cells. Cytotherapy 13(8), 1000–1012 (2011)
K.H. Nam, A.S. Smith, S. Lone, S. Kwon, D.H. Kim, Biomimetic 3D tissue models for advanced high-throughput drug screening. J Lab Autom 20(3), 201–215 (2015)
F. Salimi, K.A. Dilmaghani, E. Alizadeh, A. Akbarzadeh and S. Davaran, Enhancing cisplatin delivery to hepatocellular carcinoma HepG2 cells using dual sensitive smart nanocomposite. Artificial cells, nanomedicine, and biotechnology:, 1–10 (2017)
D.H. Shin, Y.J. Choi, J.W. Park, SIRT1 and AMPK mediate hypoxia-induced resistance of non–small cell lung cancers to cisplatin and doxorubicin. Cancer Res. 74(1), 298–308 (2014)
S. Surget, M.P. Khoury, J.C. Bourdon, Uncovering the role of p53 splice variants in human malignancy: A clinical perspective. Onco Targets Ther 7, 57 (2014)
M.W. Tibbitt, K.S. Anseth, Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 103(4), 655–663 (2009)
Y.C. Tung, A.Y. Hsiao, S.G. Allen, Y.S. Torisawa, M. Ho, S. Takayama, High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 136(3), 473–478 (2011)
C. Veelken, G.J. Bakker, D. Drell, P. Friedl, Single cell-based automated quantification of therapy responses of invasive cancer spheroids in organotypic 3D culture. Methods 128, 139–149 (2017)
K. von der Mark, V. Gauss, H. von der Mark, P. Müller, Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 267(5611), 531 (1977)
X.J. Wang, Y. Li, L. Luo, H. Wang, Z. Chi, A. Xin, X. Li, J. Wu, X. Tang, Oxaliplatin activates the Keap1/Nrf2 antioxidant system conferring protection against the cytotoxicity of anticancer drugs. Free Radic. Biol. Med. 70, 68–77 (2014)
L. Zou, C. Wu, Q. Wang, J. Zhou, K. Su, H. Li, N. Hu, P. Wang, An improved sensitive assay for the detection of PSP toxins with neuroblastoma cell-based impedance biosensor. Biosens. Bioelectron. 67, 458–464 (2015)
Acknowledgements
This work was supported by National Natural Science Fund of China (No. 61320106002, 31627801, 31661143030) and National 973 project (No. 2015CB352101).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(JPEG 49 kb)
Fig. S1 Long term culture of A549 spheroid form 1 to 5 days (a-e), cells assembled more closely and the surface became smooth. f. Live/dead staining of A549 spheroid. This fluorescent merged images present high viability of spheroid in day 5. Scale bar 300 μm.
ESM 2
(JPEG 113 kb)
Fig. S2 Determination of cells number for 2D culture control. A group of representative cell growth curves of A549 (a) and H1299 (b) cells at different cell numbers ranging from 1250 to 20000 cells/well. The linear relationship of A549 (c) and H1299 (d) cells between the cell number and the CI value at 12h after cells were seeded.
ESM 3
(JPEG 64.3 kb)
Fig. S3 Cisplatin, etoposide and pemetrexed with the concentration of 10 μM, 10 μM and 100 μM, respectively, were used to detect the impedance for 12h as well as the medium control. All anticancirogens were diluted by standard medium as they treat to spheroids.
ESM 4
(JPEG 195 kb)
Fig. S4 a. The multi-organs-on-a-chip platform linked through silicone tube and cycled byperistaltic pump. b. Fluid with drug traversed to drug well, HL-1, A549 and HepG2 successively.
Rights and permissions
About this article
Cite this article
Wu, Q., Wei, X., Pan, Y. et al. Bionic 3D spheroids biosensor chips for high-throughput and dynamic drug screening. Biomed Microdevices 20, 82 (2018). https://doi.org/10.1007/s10544-018-0329-x
Published:
DOI: https://doi.org/10.1007/s10544-018-0329-x