Abstract
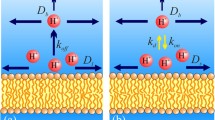
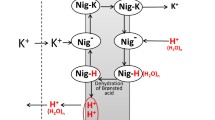
Proton transfer between water and the interior of membrane proteins plays a key role in bioenergetics. Here we survey the mechanism of this transfer as inferred from experiments with flash-triggered enzymes capturing or ejecting protons at the membrane surface. These experiments have revealed that proton exchange between the membrane surface and the bulk water phase proceeds at ≥1 msec because of a kinetic barrier for electrically charged species. From the data analysis, the barrier height for protons could be estimated as about 0.12 eV, i.e., high enough to account for the observed retardation in proton exchange. Due to this retardation, the proton activity at the membrane surface might deviate, under steady turnover of proton pumps, from that measured in the adjoining water phase, so that the driving force for ATP synthesis might be higher than inferred from the bulk-to-bulk measurements. This is particularly relevant for alkaliphilic bacteria. The proton diffusion along the membrane surface, on the other hand, is unconstrained and fast, occurring between the neighboring enzymes at less than 1 µsec. The anisotropy of proton dynamics at the membrane surface helps prokaryotes diminish the “futile” escape of pumped protons into the external volume. In some bacteria, the inner membrane is invaginated, so that the “ejected ” pro tons get trapped in the closed space of such intracellular membrane “sacks” which can be round or flat. The chloroplast thylakoids and the mitochondrial cristae have their origin in these intracellular structures.
Similar content being viewed by others
REFERENCES
Mitchell, P. (1961) Nature, 191, 144–148.
Mitchell, P. (1966) Physiol. Rev., 41, 445–502.
Skulachev, V. P. (1977) FEBS Lett., 74, 1–9.
Skulachev, V. P. (1992) Eur. J. Biochem., 208, 203–209.
Williams, R. J. P. (1978) Biochim. Biophys. Acta, 505, 1–44.
Krulwich, T. A., Ito, M., Gilmour, R., Sturr, M. G., Guffanti, A. A., and Hicks, D. B. (1996) Biochim. Biophys. Acta, 1275, 21–26.
Guffanti, A. A., Mann, M., Sherman, T. L., and Krulwich, T. A. (1984) J. Bacteriol., 159, 448–452.
Ferguson, S. J. (1985) Biochim. Biophys. Acta, 811, 47–95.
Cramer, W. A., and Knaff, D. B. (1991) Energy Transduction in Biological Membranes: a Textbook of Bioenergetics, Springer, New York.
Ferguson, S. J. (1995) Curr. Biol., 5, 25–27.
Kell, D. B. (1979) Biochim. Biophys. Acta, 549, 55–99.
Michel, H., and Oesterhelt, D. (1980) Biochemistry, 19, 4615–4619.
Guffanti, A. A., and Krulwich, T. A. (1984) Biochem. Soc. Trans., 12, 411–412.
Kell, D. B. (1986) Meth. Enzymol., 127, 538–557.
Eigen, M. (1963) Angew. Chem., 75, 489–588.
Wraight, C. A., Cogdell, R. J., and Chance, B. (1978) in The Photosynthetic Bacteria (Clayton, R. K., and Sistrom, W. R., eds.) Academic Press, New York, pp. 471–511.
Junge, W., and Jackson, J. B. (1982) in Photosynthesis (Govindjee, ed.) Vol. 1, Academic Press, New York, pp. 589–646.
Chance, B., Crofts, A. R., Nishimura, M., and Price, B. (1970) Eur. J. Biochem., 13, 364–374.
Codgell, R. J., Jackson, J. B., and Crofts, A. R. (1972) Bioenerg., 4, 413–429.
Petty, K. M., and Dutton, P. L. (1976) Arch. Biochem. Biophys., 172, 335–345.
Auslander, W., and Junge, W. (1974) Biochim. Biophys. Acta, 357, 285–298.
Drachev, A. L., Kaulen, A. D., and Skulachev, V. P. (1984) FEBS Lett., 178, 331–336.
Heberle, J., and Dencher, N. A. (1990) FEBS Lett., 277, 277–280.
Heberle, J., and Dencher, N. A. (1992) Proc. Natl. Acad. Sci. USA, 89, 5996–6000.
Heberle, J., Riesle, J., Thiedemann, G., Oesterhelt, D., and Dencher, N. A. (1994) Nature, 370, 379–382.
Scherrer, P., Alexiev, U., Marti, T., Khorana, H. G., and Heyn, M. P. (1994) Biochemistry, 33, 13684–13692.
Dioumaev, A. K., Richter, H. T., Brown, L. S., Tanio, M., Tuzi, S., Saito, H., Kimura, Y., Needleman, R., and Lanyi, J. K. (1998) Biochemistry, 37, 2496–2506.
Porschke, D. (2002) J. Phys. Chem. B, 106, 10233–10241.
Gopta, O. A., Cherepanov, D. A., Junge, W., and Mulkidjanian, A. Y. (1999) Proc. Natl. Acad. Sci. USA, 96, 13159–13164.
Junge, W., and Polle, A. (1986) Biochim. Biophys. Acta, 848, 265–273.
Heberle, J., and Dencher, N. A. (1992) in Structures and Functions of Retinal Proteins (Rigaud, J. L., ed.) John Libbey Eurotext Ltd, pp. 221–224.
Junge, W., and McLaughlin, S. (1987) Biochim. Biophys. Acta, 890, 1–5.
Jones, M. R., and Jackson, J. B. (1989) Biochim. Biophys. Acta, 975, 34–43.
Nachliel, E., and Gutman, M. (1996) FEBS Lett., 393, 221–225.
Georgievskii, Y., Medvedev, E. S., and Stuchebrukhov, A. A. (2002) Biophys. J., 82, 2833–2846.
Riesle, J., Oesterhelt, D., Dencher, N. A., and Heberle, J. (1996) Biochemistry, 35, 6635–6643.
Adelroth, P., and Brzezinski, P. (2004) Biochim. Biophys. Acta, 1655, 102–115.
Cherepanov, D. A., Junge, W., and Mulkidjanian, A. Y. (2004) Biophys. J., 86, 665–680.
Heberle, J. (1991) Zeitauflosende Untersuchung der Protonentranslokationsschritte von bakteriorhodopsin mittels chemisch-gekoppelter pH-Indikatoren, PhD Thesis, Freien Universitat, Berlin.
Cherepanov, D. A., Feniouk, B. A., Junge, W., and Mulkidjanian, A. Y. (2003) Biophys. J., 85, 1307–1316.
Cherepanov, D. A. (2005) Phys. Rev. Lett., in press.
Maroti, P., and Wraight, C. A. (1997) Biophys. J., 73, 367–381.
Arata, H., Takenaka, I., and Nishimura, M. (1987) J. Biochem., 101, 261–265.
Jones, M. R., and Jackson, J. B. (1990) Biochim. Biophys. Acta, 1019, 51–58.
Mulkidjanian, A. Y., and Junge, W. (1994) FEBS Lett., 353, 189–193.
Kramer, D. M., Sacksteder, C. A., and Cruz, J. A. (1999) Photosynth. Res., 60, 151–163.
Skulachev, V. P. (2001) Trends Biochem. Sci., 26, 23–29.
Heberle, J. (2000) Biochim. Biophys. Acta, 1458, 135–147.
Alexiev, U., Mollaaghababa, R., Scherrer, P., Khorana, H. G., and Heyn, M. P. (1995) Proc. Natl. Acad. Sci. USA, 92, 372–376.
Serowy, S., Saparov, S. M., Antonenko, Y. N., Kozlovsky, W., Hagen, V., and Pohl, P. (2003) Biophys. J., 84, 1031–1037.
Amchenkova, A. A., Bakeeva, L. E., Chentsov, Y. S., Skulachev, V. P., and Zorov, D. B. (1988) J. Cell Biol., 107, 481–495.
Severina, I. I., Skulachev, V. P., and Zorov, D. B. (1988) J. Cell Biol., 107, 497–501.
Feniouk, B. A., Kozlova, M. A., Knorre, D. A., Cherepanov, D. A., Mulkidjanian, A. Y., and Junge, W. (2004) Biophys. J., 86, 4094–4109.
Cherepanov, D. A., Mulkidjanian, A. Y., and Junge, W. (1999) FEBS Lett., 449, 1–6.
Andersson, S. G. E., Zomorodipour, A., Andersson, J. O., Sicheritz-Ponten, T., Alsmark, U. C. M., Podowski, R. M., Naslund, A. K., Eriksson, A. S., Winkler, H. H., and Kurland, C. G. (1998) Nature, 396, 133–140.
Kozlova, M. V., Gramadskii, K. B., Solodovnikova, I. M., Krasinskaya, I. P., Vinogradov, A. V., and Yaguzhinskii, L. S. (2003) Biofizika, 48, 443–452.
Solodovnikova, I. M., Yurkov, V. I., Ton’shin, A. A., and Yaguzhinskii, L. S. (2004) Biofizika, 49, 47–56.
Mitchell, P. (1991) Biosci. Rep., 11, 297–344.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Biokhimiya, Vol. 70, No. 2, 2005, pp. 308–314.
Original Russian Text Copyright © 2005 by Mulkidjanian, Cherepanov, Heberle, Junge.
Rights and permissions
About this article
Cite this article
Mulkidjanian, A.Y., Cherepanov, D.A., Heberle, J. et al. Proton transfer dynamics at membrane/water interface and mechanism of biological energy conversion. Biochemistry (Moscow) 70, 251–256 (2005). https://doi.org/10.1007/s10541-005-0108-1
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10541-005-0108-1