Abstract
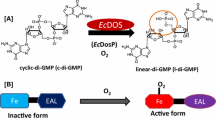
The heme-based oxygen-sensor phosphodiesterase from Escherichia coli (Ec DOS), is composed of an N-terminal heme-bound oxygen sensing domain and a C-terminal catalytic domain. Oxygen (O2) binding to the heme Fe(II) complex in Ec DOS substantially enhances catalysis. Addition of hydrogen sulfide (H2S) to the heme Fe(III) complex in Ec DOS also remarkably stimulates catalysis in part due to the heme Fe(III)–SH and heme Fe(II)–O2 complexes formed by H2S. In this study, we examined the roles of the heme distal amino acids, M95 (the axial ligand of the heme Fe(II) complex) and R97 (the O2 binding site in the heme Fe(II)–O2 complex) of the isolated heme-binding domain of Ec DOS (Ec DOS-PAS) in the binding of H2S under aerobic conditions. Interestingly, R97A and R97I mutant proteins formed an oxygen-incorporated modified heme, verdoheme, following addition of H2S combined with H2O2 generated by the reactions. Time-dependent mass spectroscopic data corroborated the findings. In contrast, H2S did not interact with the heme Fe(III) complex of M95H and R97E mutants. Thus, M95 and/or R97 on the heme distal side in Ec DOS-PAS significantly contribute to the interaction of H2S with the Fe(III) heme complex and also to the modification of the heme Fe(III) complex with reactive oxygen species. Importantly, mutations of the O2 binding site of the heme protein converted its function from oxygen sensor to that of a heme oxygenase. This study establishes the novel role of H2S in modifying the heme iron complex to form verdoheme with the aid of reactive oxygen species.






Similar content being viewed by others
Abbreviations
- Ec DOS:
-
A heme-based oxygen-sensor phosphodiesterase from Escherichia coli
- Ec DOS-PAS:
-
Isolated heme-bound PAS domain of Ec DOS
- H2S:
-
Hydrogen sulfide
- Heme Fe(II) complex:
-
Protoporphyrin IX ferrous complex
- Heme Fe(III) complex:
-
Protoporphyrin IX ferric complex
- MALDI TOF MS:
-
Matrix-assisted laser desorption/ionization
- MS:
-
Mass spectrometry
- PAS:
-
An acronym formed from Per (Drosophila period clock protein)-Arnt (vertebrate aryl hydrocarbon receptor nuclear translocator)-Sim (Drosophila single-minded protein)
- SOD:
-
Superoxide dismutase
References
Andersson LA, Loehr TM, Lim AR, Mauk AG (1984) Sulfmyoglobin. Resonance Raman spectroscopic evidence for an iron-chlorin prosthetic group. J Biol Chem 259:15340–15349
Bailly X, Vinogradov S (2005) The sulfide binding function of annelid hemoglobins: relic of an old biosystem? J Inorg Biochem 99:142–150
Banerjee R (2012) Hydrogen sulfide: redox metabolism and signaling. Antioxid Redox Signal 17:45–57
Berzofsky JA, Peisach J, Blumber WE (1971a) Sulfheme proteins. I. Optical and magnetic properties of sulfmyoglobin and its derivatives. J Biol Chem 246:3367–3377
Berzofsky JA, Peisach J, Blumberg WE (1971b) Sulfheme proteins. II. The reversible oxygenation of ferrous sulfmyoglobin. J Biol Chem 246:7366–7372
Evans SV, Sishta BP, Mauk AG, Brayer GD (1994) Three-dimensional structure of cyanomet-sulfmyoglobin C. Proc Nat Acad Sci USA 91:4723–4726
Hewson WD, Hager LP (1979) Oxidation of horseradish peroxidase compound II to compound I. J Biol Chem 254:3182–3186
Hirata S, Matsui T, Sasakura Y, Sugiyama S, Yoshimura T, Sagami I, Shimizu T (2003) Characterization of Met95 mutants of a heme-regulated phosphodiesterase from Escherichia coli: optical absorption, magnetic circular dichroism, circular dichroism, and redox potentials. Eur J Biochem 270:4771–4779
Igarashi J, Kitanishi K, Shimizu T (2011) Emerging role of heme as a signal and the gas-sensing site: heme-sensing and gas-sensing proteins. In: Kadish KM, Smith KM, Guilard R (eds) Handbook of porphyrin science, vol 15. World Scientific Hackensack, USA, pp 399–460
Ishitsuka Y, Araki Y, Tanaka A, Igarashi J, Ito O, Shimizu T (2008) Arg97 at the heme-distal side of the isolated heme-bound PAS domain of a heme-based oxygen sensor from Escherichia coli (Ec DOS) plays critical roles in autooxidation and binding of gases, particularly O2. Biochemistry 47:8874–8884
Kabil O, Banerjee R (2010) Redox biochemistry of hydrogen sulfide. J Biol Chem 285:21903–21907
Kimura H, Shibuya N, Kimura Y (2012) Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antioxid Redox Signal 17:45–57
Kitanishi K, Kobayashi K, Kawamura Y, Ishigami I, Ogura T, Nakajima K, Igarashi J, Tanaka A, Shimizu T (2010) Important roles of Tyr43 at the putative heme distal side in the oxygen recognition and stability of the Fe(II)-O2 complex of YddV, a globin-coupled heme-based oxygen sensor diguanylate cyclase. Biochemistry 49:10381–10393
Kitanishi K, Kobayashi K, Uchida T, Ishimori K, Igarashi J, Shimizu T (2011) Identification and functional and spectral characterization of a globin-coupled histidine kinase from Anaeromyxobacter sp. Fw109-5. J Biol Chem 286:35522–35534
Kraus DW, Wittenberg JB (1990) Hemoglobins of the Lucina pectinata/bacteria symbiosis. I. Molecular properties, kinetics and equilibria of reactions with ligands. J Biol Chem 265:16043–16053
Kraus DW, Wittenberg JB, Jin-Fen L, Peisach J (1990) Hemoglobins of the Lucina pectinata/bateria symbiosis. II. An electron paramagnetic resonance and optical spectral study of the ferric proteins. J Biol Chem 265:16054–16059
Kurokawa H, Lee DS, Watanabe M, Sagami I, Mikami B, Raman CS, Shimizu T (2004) A redox-controlled molecular switch revealed by the crystal structure of a bacterial heme PAS sensor. J Biol Chem 279:20186–20193
Lloyd E, Mauk AG (1994) Formation of sulphmyoglobin during expression of horse heart myoglobin in Escherichia coli. FEBS Lett 340:281–286
Matsui T, Ozaki S, Watanabe Y (1997) On the formation and reactivity of compound I of the His-64 myoglobin mutants. J Biol Chem 272:32735–32738
Matsui T, Iwasaki M, Sugiyama R, Unno M, Ikeda-Saito M (2010) Dioxygen activation for the self-degradation of heme: reaction mechanism and regulation of heme oxygenase. Inorg Chem 49:3602–3609
Nicoletti FP, Comandini A, Bonamore A, Boechi L, Boubeta FM, Feis A, Smulvich G, Boffi A (2010) Sulfide binding properties of truncated hemoglobin. Biochemistry 49:2269–2278
Ortiz de Montellano PR (1998) Heme oxygenase mechanistic evidence for an electrophilic ferric peroxide species. Acc Chem Res 31:543–549
Park H, Suquet C, Satterlee JD, Kang C (2004) Insights into signal transduction involving PAS domain oxygen-sensing heme proteins from the X-ray crystal structure of Escherichia coli DOS heme domain (Ec DOSH). Biochemistry 43:2738–2746
Paul BD, Snyder SH (2012) H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13:499–507
Pietri R, Lewis A, León RG, Casanoba G, Kiger L, Yeh SR, Ferdandez-Alberti S, Marden MC, Cadilla CL, López-Garriga J (2009) Factors controlling the reactivity of hydrogen sulfide with hemeproteins. Biochemistry 48:4881–4891
Pietri R, Román-Morales E, López-Garriga J (2011) Hydrogen sulfide and hemeproteins: knowledge and mysteries. Antioxid Redox Signal 15:393–404
Ragsdale SW, Yi L (2011) Thiol/disulfide redox switches in the regulation of heme binding to proteins. Antioxid Redox Signal 14:1039–1047
Román-Morales E, Pietri R, Ramos-Santana B, Vinogradov SN, Lewis-Ballester A, López-Garriga J (2010) Structural determinants for the formation of sulfhemeprotein complexes. Biochem Biophys Res Commun 400:489–492
Sakamoto H, Omata Y, Adachi Y, Palmer G, Noguchi M (2000) Separation and identification of regioisomers of verdoheme by reverse-phase ion-pair high-performance liquid chromatography, and characterization of their complexes with heme oxygenase. J Inorg Biochem 82:113–121
Sasakura Y, Yoshimura-Suzuki T, Kurokawa H, Shimizu T (2006) Structure-function relationships of EcDOS, a heme-regulated phosphodiesterase from Escherichia coli. Acc Chem Res 39:37–43
Shikama K (1998) The molecular mechanism of autooxidation for myoglobin and hemoglobin: a venerable puzzle. Chem Rev 98:1357–1374
Shimizu T (2012) Binding of cysteine thiolate to the Fe(III) heme complex is critical for the function of heme sensor proteins. J Inorg Biochem 108:171–177
Springer BA, Sligar SG, Olson JS, Phillips GN Jr (1994) Mechanisms of ligand recognition in myoglobin. Chem Rev 94:699–714
Sugishima M, Moffat K, Noguchi M (2012) Discrimination between CO and O2 in heme oxygenase: comparison of static structures and dynamic conformation changes following CO photolysis. Biochemistry 51:8554–8562
Szabo C (2007) Hydrogen sulphide and its therapeutic potential. Nature Rev Drug Disco 6:917–935
Szabo C (2010) Gaseotransmitters: new frontiers for translational science. Sci Transl Med 2(59):ps54
Taguchi S, Matsui T, Igarashi J, Sasakura Y, Araki Y, Ito O, Sugiyama S, Sagami I, Shimizu T (2004) Binding of oxygen and carbon monoxide to a heme-regulated phosphodiesterase from Escherichia coli: kinetics and infrared spectra of the full-length wild-type enzyme, isolated PAS domain, and Met95 mutants. J Biol Chem 279:3340–3347
Takahashi H, Sekimoto M, Tanaka M, Tanaka A, Igarashi J, Shimizu T (2012) Hydrogen sulfide stimulates the catalytic activity of a heme-regulated phosphodiesterase from Escherichia coli (Ec DOS). J Inorg Biochem 109:66–71
Tanaka A, Shimizu T (2008) Ligand binding to the Fe(III)-protoporphyrin IX complex of phosphodiesterase from Escherichia coli (Ec DOS) markedly enhances catalysis of cyclic di-GMP: roles of Met95, Arg97, and Phe113 of the putative heme distal side in catalytic regulation and ligand binding. Biochemistry 47:13438–13446
Tanaka A, Takahashi H, Shimizu T (2007) Critical role of the heme axial ligand, Met95, in locking catalysis of the phosphodiesterase from Escherichia coli (Ec DOS) toward cyclic diGMP. J Biol Chem 282:21301–21307
Wang R (2010) Hydrogen sulfide: the third gaseotransmitter in biology and medicine. Antioxid Redox Signal 12:1061–1064
Watanabe M, Matsui T, Sasakura Y, Sagami I, Shimizu T (2002) Unusual cyanide bindings to a heme-regulated phosphodiesterase from Escherichia coli: effect of Met95 mutations. Biochem Biophys Res Commun 299:169–172
Acknowledgments
This work was in part supported by Grants-in-Aid from Shantou University Medical College and from the National Natural Science Foundation of China (NSFC) (No. 31170736) (to T. S.), and by Charles University in Prague (UNCE 204025/2012) (to M. M.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Du, Y., Liu, G., Yan, Y. et al. Conversion of a heme-based oxygen sensor to a heme oxygenase by hydrogen sulfide: effects of mutations in the heme distal side of a heme-based oxygen sensor phosphodiesterase (Ec DOS). Biometals 26, 839–852 (2013). https://doi.org/10.1007/s10534-013-9640-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-013-9640-4