Abstract
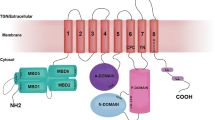
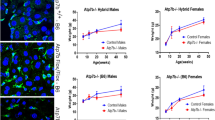
Copper-transporting ATPase ATP7B (Wilson disease protein) is a member of the P-type ATPase family with characteristic domain structure and distinct ATP-binding site. ATP7B plays a central role in the regulation of copper homeostasis in the liver by delivering copper to the secretory pathway and mediating export of excess copper into the bile. The dual function of ATP7B in hepatocytes is coupled with copper-dependent intracellular relocalization of the transporter. The final destination of ATP7B in hepatocytes during the copper-induced trafficking process is still under debate. We show the results of immunocytochemistry experiments in polarized HepG2 cells that support the model in which elevated copper induces trafficking of ATP7B to sub-apical vesicles, and transiently to the canalicular membrane. In Atp7b -/- mice, an animal model of Wilson disease, both copper delivery to the trans-Golgi network and copper export into the bile are disrupted despite large accumulation of copper in the cytosol. We review the biochemical and physiological changes associated with Atp7b inactivation in mouse liver and discuss the pleiotropic consequences of the common Wilson disease mutation, His1069Gln.



Similar content being viewed by others
References
Linder MC, Wooten L, Cerveza P, Cotton S, Shulze R, Lomeli N (1998) Copper transport. Am J Clin Nutr 67:965S–971S
Wijmenga C, Klomp LW (2004) Molecular regulation of copper excretion in the liver. Proc Nutr Soc 63:31–9
Prohaska JR, Gybina AA (2004) Intracellular copper transport in mammals. J Nutr 134:1003–6
Puig S, Thiele DJ (2002) Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol 6:171–80
O’Halloran TV, Culotta VC (2000) Metallochaperones, an intracellular shuttle service for metal ions. J Biol Chem 275:25057–60
Xiao Z, Wedd AG (2002) A C-terminal domain of the membrane copper pump Ctr1 exchanges copper(I) with the copper chaperone Atx1. Chem Commun (Camb) 588–9
Arnesano F, Banci L, Bertini I, Martinelli M (2005) Ortholog search of proteins involved in copper delivery to cytochrome C oxidase and functional analysis of paralogs and gene neighbors by genomic context. J Proteome Res 4:63–70
Horng YC, Cobine PA, Maxfield AB, Carr HS, Winge DR (2004) Specific copper transfer from the Cox17 metallochaperone to both Sco1 and Cox11 in the assembly of yeast cytochrome C oxidase. J Biol Chem 279:35334–40
Leary SC, Kaufman BA, Pellecchia G, Guercin GH, Mattman A, Jaksch M, Shoubridge EA (2004) Human SCO1 and SCO2 have independent, cooperative functions in copper delivery to cytochrome c oxidase. Hum Mol Genet 13:1839–48
Bartnikas TB, Gitlin JD (2003) Mechanisms of biosynthesis of mammalian copper/zinc superoxide dismutase. J Biol Chem 278:33602–8
Casareno RL, Waggoner D, Gitlin JD (1998) The copper chaperone CCS directly interacts with copper/zinc superoxide dismutase. J Biol Chem 273:23625–8
Wong PC, Waggoner D, Subramaniam JR, Tessarollo L, Bartnikas TB, Culotta VC, Price DL, Rothstein J, Gitlin JD (2000) Copper chaperone for superoxide dismutase is essential to activate mammalian Cu/Zn superoxide dismutase. Proc Natl Acad Sci U S A 97:2886–91
Hung IH, Casareno RL, Labesse G, Mathews FS, Gitlin JD (1998) HAH1 is a copper-binding protein with distinct amino acid residues mediating copper homeostasis and antioxidant defense. J Biol Chem 273:1749–54
Klomp LW, Lin SJ, Yuan DS, Klausner RD, Culotta VC, Gitlin JD (1997) Identification and functional expression of HAH1, a novel human gene involved in copper homeostasis. J Biol Chem 272:9221–6
Murata Y, Yamakawa E, Iizuka T, Kodama H, Abe T, Seki Y, Kodama M (1995) Failure of copper incorporation into ceruloplasmin in the Golgi apparatus of LEC rat hepatocytes. Biochem Biophys Res Commun 209:349–55
Terada K, Kawarada Y, Miura N, Yasui O, Koyama K, Sugiyama T (1995) Copper incorporation into ceruloplasmin in rat livers. Biochim Biophys Acta 1270:58–62
Hellman NE, Kono S, Mancini GM, Hoogeboom AJ, De Jong GJ, Gitlin JD (2002) Mechanisms of copper incorporation into human ceruloplasmin. J Biol Chem 277:46632–8
Langner C, Denk H (2004) Wilson disease. Virchows Arch 445:111–8
Lutsenko S, Efremov RG, Tsivkovskii R, Walker JM (2002) Human copper-transporting ATPase ATP7B (the Wilson’s disease protein): biochemical properties and regulation. J Bioenerg Biomembr 34:351–62
Tsivkovskii R, MacArthur BC, Lutsenko S (2001) The Lys1010-Lys1325 fragment of the Wilson’s disease protein binds nucleotides and interacts with the N-terminal domain of this protein in a copper-dependent manner. J Biol Chem 276:2234–42
Bissig KD, Wunderli-Ye H, Duda PW, Solioz M (2001) Structure-function analysis of purified Enterococcus hirae CopB copper ATPase: effect of Menkes/Wilson disease mutation homologues. Biochem J 357:217–23
Forbes JR, Cox DW (1998) Functional characterization of missense mutations in ATP7B: Wilson disease mutation or normal variant? Am J Hum Genet 63:1663–74
Mandal AK, Arguello JM (2003) Functional roles of metal binding domains of the Archaeoglobus fulgidus Cu(+)-ATPase CopA. Biochemistry 42:11040–7
Mandal AK, Yang Y, Kertesz TM, Arguello JM (2004) Identification of the transmembrane metal binding site in Cu+-transporting PIB-type ATPases. J Biol Chem 279:54802–7
Voskoboinik I, Greenough M, La Fontaine S, Mercer JF, Camakaris J (2001) Functional studies on the Wilson copper P-type ATPase and toxic milk mouse mutant. Biochem Biophys Res Commun 281:966–70
Walker JM, Tsivkovskii R, Lutsenko S (2002) Metallochaperone Atox1 transfers copper to the NH2–terminal domain of the Wilson’s disease protein and regulates its catalytic activity. J Biol Chem 277:27953–9
Larin D, Mekios C, Das K, Ross B, Yang AS, Gilliam TC (1999) Characterization of the interaction between the Wilson and Menkes disease proteins and the cytoplasmic copper chaperone, HAH1p. J Biol Chem 274:28497–504
Arnesano F, Banci L, Bertini I, Ciofi-Baffoni S, Molteni E, Huffman DL, O’Halloran TV (2002) Metallochaperones and metal-transporting ATPases: a comparative analysis of sequences and structures. Genome Res 12:255–71
DiDonato M, Hsu HF, Narindrasorasak S, Que L Jr, Sarkar B (2000) Copper-induced conformational changes in the N-terminal domain of the Wilson disease copper-transporting ATPase. Biochemistry 39:1890–6
Ralle M, Lutsenko S, Blackburn NJ (2004) Copper transfer to the N-terminal domain of the Wilson disease protein (ATP7B): X-ray absorption spectroscopy of reconstituted and chaperone-loaded metal binding domains and their interaction with exogenous ligands. J Inorg Biochem 98:765–74
Walker JM, Huster D, Ralle M, Morgan CT, Blackburn NJ, Lutsenko S (2004) The N-terminal metal-binding site 2 of the Wilson’s Disease Protein plays a key role in the transfer of copper from Atox1. J Biol Chem 279:15376–84
Bunce J, Achila D, Hetrick E, Lesley L, Huffman DL (2006) Copper transfer studies between the N-terminal copper binding domains one and four of human Wilson protein. Biochim Biophys Acta 1760:907–12
Huster D, Lutsenko S (2003) The distinct roles of the N-terminal copper-binding sites in regulation of catalytic activity of the Wilson’s disease protein. J Biol Chem 278:32212–8
Cater MA, Forbes J, La Fontaine S, Cox D, Mercer JF (2004) Intracellular trafficking of the human Wilson protein: the role of the six N-terminal metal-binding sites. Biochem J 380:805–13
Guo Y, Nyasae L, Braiterman LT, Hubbard AL (2005) NH2-terminal signals in ATP7B Cu-ATPase mediate its Cu-dependent anterograde traffic in polarized hepatic cells. Am J Physiol Gastrointest Liver Physiol 289:G904–16
Achila D, Banci L, Bertini I, Bunce J, Ciofi-Baffoni S, Huffman DL (2006) Structure of human Wilson protein domains 5 and 6 and their interplay with domain 4 and the copper chaperone HAH1 in copper uptake. Proc Natl Acad Sci U S A 103:5729–34
Tsivkovskii R, Eisses JF, Kaplan JH, Lutsenko S (2002) Functional properties of the copper-transporting ATPase ATP7B (the Wilson’s disease protein) expressed in insect cells. J Biol Chem 277:97683
Tao TY, Liu F, Klomp L, Wijmenga C, Gitlin JD (2003) The copper toxicosis gene product Murr1 directly interacts with the Wilson disease protein. J Biol Chem 278:41593–6
Lim CM, Cater MA, Mercer JF, La Fontaine S (2006) Copper-dependent interaction of dynactin subunit p62 with the N terminus of ATP7B but not ATP7A. J Biol Chem 281:14006–14
Lim CM, Cater MA, Mercer JF, La Fontaine S (2006) Copper-dependent interaction of glutaredoxin with the N termini of the copper-ATPases (ATP7A and ATP7B) defective in Menkes and Wilson diseases. Biochem Biophys Res Commun 348:428–36
Toyoshima C, Mizutani T (2004) Crystal structure of the calcium pump with a bound ATP analogue. Nature 430:529–35
Sazinsky MH, Mandal AK, Arguello JM, Rosenzweig AC (2006) Structure of the ATP binding domain from the Archaeoglobus fulgidus Cu+-ATPase. J Biol Chem 281:11161–6
Dmitriev O, Tsivkovskii R, Abildgaard F, Morgan CT, Markley JL, Lutsenko S (2006) Solution structure of the N-domain of Wilson disease protein: distinct nucleotide-binding environment and effects of disease mutations. Proc Natl Acad Sci U S A 103:5302–7
Sazinsky MH, Agarwal S, Arguello JM, Rosenzweig AC (2006) Structure of the Actuator Domain from the Archaeoglobus fulgidus Cu(+)-ATPase(,). Biochemistry 45:9949–9955
Anthonisen AN, Clausen JD, Andersen JP (2006) Mutational analysis of the conserved TGES loop of sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem 281:31572–82
Toyoshima C, Inesi G (2004) Structural basis of ion pumping by Ca2+-ATPase of the sarcoplasmic reticulum. Annu Rev Biochem 73:269–92
Moller JV, Olesen C, Jensen AM, Nissen P (2005) The structural basis for coupling of Ca2+ transport to ATP hydrolysis by the sarcoplasmic reticulum Ca2+-ATPase. J Bioenerg Biomembr 37:359–64
Paulsen M, Lund C, Akram Z, Winther JR, Horn N, Moller LB (2006) Evidence that translation reinitiation leads to a partially functional menkes protein containing two copper-binding sites. Am J Hum Genet 79:214–29
Majumdar R, Al Jumah M, Al Rajeh S, Fraser M, Al Zaben A, Awada A, Al Traif I, Paterson M (2000) A novel deletion mutation within the carboxyl terminus of the copper-transporting ATPase gene causes Wilson disease. J Neurol Sci 179:140–3
Hsi G, Cullen LM, Moira Glerum D, Cox DW (2004) Functional assessment of the carboxy-terminus of the Wilson disease copper-transporting ATPase, ATP7B. Genomics 83:473–81
Cater MA, La Fontaine S, Shield K, Deal Y, Mercer JF (2006) ATP7B mediates vesicular sequestration of copper: insight into biliary copper excretion. Gastroenterology 130:493–506
Schaefer M, Hopkins RG, Failla ML, Gitlin JD (1999) Hepatocyte-specific localization and copper-dependent trafficking of the Wilson’s disease protein in the liver. Am J Physiol 276:G639–46
Vanderwerf SM, Cooper MJ, Stetsenko IV, Lutsenko S (2001) Copper specifically regulates intracellular phosphorylation of the Wilson’s disease protein, a human copper-transporting ATPase. J Biol Chem 276:36289–94
Hung IH, Suzuki M, Yamaguchi Y, Yuan DS, Klausner RD, Gitlin JD (1997) Biochemical characterization of the Wilson disease protein and functional expression in the yeast Saccharomyces cerevisiae. J Biol Chem 272:21461–6
Roelofsen H, Wolters H, Van Luyn MJ, Miura N, Kuipers F, Vonk RJ (2000) Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology 119:782–93
Fanni D, Pilloni L, Orru S, Coni P, Liguori C, Serra S, Lai ML, Uccheddu A, Contu L, Van Eyken P et al (2005) Expression of ATP7B in normal human liver. Eur J Histochem 49:371–8
Payne AS, Kelly EJ, Gitlin JD (1998) Functional expression of the Wilson disease protein reveals mislocalization and impaired copper dependent trafficking of the common H1069Q mutation. Proc Natl Acad Sci U S A 95:10854–9
Huster D, Hoppert M, Lutsenko S, Zinke J, Lehmann C, Mossner J, Berr F, Caca K (2003) Defective cellular localization of mutant ATP7B in Wilson’s disease patients and hepatoma cell lines. Gastroenterology 124:335–45
Tsivkovskii R, Efremov RG, Lutsenko S (2003) The role of the invariant His-1069 in folding and function of the Wilson’s disease protein, the human copper-transporting ATPase ATP7B. J Biol Chem 278:13302–8
Morgan CT, Tsivkovskii R, Kosinsky YA, Efremov RG, Lutsenko S (2004) The distinct functional properties of the nucleotide-binding domain of ATP7B, the human copper-transporting ATPase: analysis of the Wilson disease mutations E1064A, H1069Q, R1151H, and C1104F. J Biol Chem 279:36363–71
Voskoboinik I, Mar J, Camakaris J (2003) Mutational analysis of the Menkes copper P-type ATPase (ATP7A). Biochem Biophys Res Commun 301:488–94
Gromadzka G, Schmidt HH, Genschel J, Bochow B, Rodo M, Tarnacka B, Litwin T, Chabik G, Czlonkowska A (2006) p.H1069Q mutation in ATP7B and biochemical parameters of copper metabolism and clinical manifestation of Wilson’s disease. Mov Disord 21:245–8
Gitlin JD (2003) Wilson disease. Gastroenterology 125:1868–77
Huster, D., Kuhn, H.J., Mossner, J. and Caca, K (2005) [Wilson disease]. Internist (Berl), 46:731–2, 734–6, 738–40
Hussain SP, Raja K, Amstad PA, Sawyer M, Trudel LJ, Wogan GN, Hofseth LJ, Shields PG, Billiar TR, Trautwein C et al (2000) Increased p53 mutation load in nontumorous human liver of wilson disease and hemochromatosis: oxyradical overload diseases. Proc Natl Acad Sci U S A 97:12770–5
Attri S, Sharma N, Jahagirdar S, Thapa BR, Prasad R (2006) Erythrocyte metabolism and antioxidant status of patients with Wilson disease with hemolytic anemia. Pediatr Res 59:593–7
Gerbasi V, Lutsenko S, Lewis EJ (2003) A mutation in the ATP7B copper transporter causes reduced dopamine beta-hydroxylase and norepinephrine in mouse adrenal. Neurochem Res 28:867–73
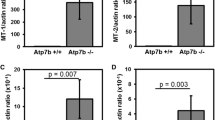
Huster D, Finegold MJ, Morgan CT, Burkhead JL, Nixon R, Vanderwerf SM, Gilliam CT, Lutsenko S (2006) Consequences of copper accumulation in the livers of the Atp7b-/- (Wilson disease gene) knockout mice. Am J Pathol 168:423–34
Buiakova OI, Xu J, Lutsenko S, Zeitlin S, Das K, Das S, Ross BM, Mekios C, Scheinberg IH, Gilliam TC (1999) Null mutation of the murine ATP7B (Wilson disease) gene results in intracellular copper accumulation and late-onset hepatic nodular transformation. Hum Mol Genet 8:1665–71
Fuentealba IC, Aburto EM (2003) Animal models of copperassociated liver disease. Comp Hepatol 2:5
Huster D, Purnat TD, Ralle M, Fiehn O, Stuckert F, Burkhead JL, Olson NE, Teupser D and S, L (2006) High copper selectively alters lipid matabolism and cell cycle (submitted).
Acknowledgements
This work was supported by the National Institute of Health grants F31-NS047963 to M.Y.B. (M.Min) and PO1 GM 067166 and R01DK071865 to S.L.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bartee, M.Y., Lutsenko, S. Hepatic copper-transporting ATPase ATP7B: function and inactivation at the molecular and cellular level. Biometals 20, 627–637 (2007). https://doi.org/10.1007/s10534-006-9074-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-006-9074-3