Abstract
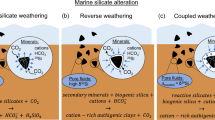
Salt marshes are sites of silica (SiO2) cycling and export to adjacent coastal systems, where silica availability can exert an important control over coastal marine primary productivity. Mineral weathering and biologic fixation concentrate silica in these systems; however, the relative contributions of geologic versus biogenic silica dissolution to this export are not known. We collected water samples from the tidal creek of a relatively undisturbed New England (USA) salt marsh over 13 tidal cycles in spring, summer, and fall 2014–2016 to determine patterns of dissolved silica (DSi) concentration in the water entering and leaving the marsh. DSi concentrations in the tidal creek peaked in the summer and were at a minimum in the fall. Additionally, we analyzed DSi concentrations and Ge/Si ratios in marsh porewater and groundwater samples as a tracer of DSi origin. Ge/Si ratios in the porewater, subterranean estuary, and fresh groundwater averaged 6.3 ± 0.31 µmol/mol, which is consistent with production via silicate weathering rather than biogenic silica dissolution. These results highlight a previously unstudied role marsh sediment plays in coastal biogeochemistry by supplying DSi to coastal ecosystems. This marsh exported 1170 mmol DSi m−2 year−1, 85% of which originated from porewater exchange, with minor contributions from brackish groundwater discharge from the subterranean estuary. Examining these values in the context of the other known DSi inputs indicates that coastal marshes provide ~ 75% of the annual silica inputs into the adjacent estuary, Waquoit Bay.







Similar content being viewed by others
Data Availability
The datasets generated and analyzed during this project are currently available as U.S. Geological Survey (USGS) data releases (https://www.sciencebase.gov/catalog/item/5fd0c4c0d34e30b91239b615; https://www.usgs.gov/data/time-series-biogeochemical-and-flow-data-tidal-salt-marsh-creek-sage-lot-pond-waquoit-bay) and on Dryad Digital Repository (https://doi.org/10.5061/dryad.zs7h44jdd).
References
Alexandre A, Meunier J-D, Colin F, Koud J-M (1997) Plant impact on the biogeochemical cycle of silicon and related weathering processes. Geochim Cosmochim Acta 61(3):677–682
Antler G, Mills JV, Hutchings AM, Redeker KR, Turchyn AV (2019) The sedimentary carbon-sulfer-iron interplay—a lesson from East Anglian salt marsh sediments. Front Earth Sci 7:140
Azam F, Volcani B (1981) Germanium-silicon interactions in biological systems. In: Silicon and siliceous structures in biological systems. Springer. p 43–67
Balke T, Stock M, Jensen K, Bouma TJ, Kleyer M (2016) A global analysis of the seaward salt marsh extent: the importance of tidal range. Water Resour Res 52(5):3775–3786
Baronas JJ, Torres MA, West AJ, Rouxel O, Georg B, Bouchez J, Gaillardet J, Hammond DE (2018) Ge and Si isotope signatures in rivers: a quantitative multi-proxy approach. Earth Planet Sci Lett 503:194–215
Boumans RM, Day JW (1993) High precision measurements of sediment elevation in shallow coastal areas using a sedimentation-erosion table. Estuaries 16(2):375–380
Brooks T, Kroeger M, Mann K, Wang A, Ganju Z, Suttles NOK, Brosnahan J, Chu S, Song S, Pohlman S (2021) Geochemical data supporting investigation of solute and particle cycling and fluxes from two tidal wetlands on the south shore of Cape Cod, Massachusetts, 2012–2019
Carey J (2020) Soil age alters the global silicon cycle. Science 369(6508):1161–1162
Carey J, Fulweiler R (2014a) Salt marsh tidal exchange increases residence time of silica in estuaries. Limnol Oceanogr 59(4):1203–1212
Carey JC, Fulweiler R (2012) Human activities directly alter watershed dissolved silica fluxes. Biogeochemistry 111(1–3):125–138
Carey JC, Fulweiler RW (2013) Nitrogen enrichment increases net silica accumulation in a temperate salt marsh. Limnol Oceanogr 58(1):99–111
Carey JC, Fulweiler RW (2014b) Silica uptake by Spartina—evidence of multiple modes of accumulation from salt marshes around the world. Front Plant Sci 5:186
Chmura GL, Anisfeld SC, Cahoon DR, Lynch JC (2003) Global carbon sequestration in tidal, saline wetland soils. Glob Biogeochem Cycles. https://doi.org/10.1029/2002GB001917
Chu SN, Wang ZA, Gonneea ME, Kroeger KD, Ganju NK (2018) Deciphering the dynamics of inorganic carbon export from intertidal salt marshes using high-frequency measurements. Mar Chem 206:7–18
Clymans W, Conley DJ, Battles JJ, Frings PJ, Koppers MM, Likens GE, Johnson CE (2016) Silica uptake and release in live and decaying biomass in a northern hardwood forest. Ecology 97(11):3044–3057
Conley DJ (2002) Terrestrial ecosystems and the global biogeochemical silica cycle. Glob Biogeochem Cycles 16(4): 68-61–68-68
Conley DJ, Malone TC (1992) Annual cycle of dissolved silicate in Chesapeake Bay: implications for the production and fate of phytoplankton biomass. Mar Ecol Prog Ser Oldendorf 81(2):121–128
Cooke J, Leishman MR (2011) Is plant ecology more siliceous than we realise? Trends Plant Sci 16(2):61–68
Cooke J, Leishman MR (2016) Consistent alleviation of abiotic stress with silicon addition: a meta-analysis. Funct Ecol 30(8):1340–1357
Cornelis J-T, Delvaux B, Cardinal D, André L, Ranger J, Opfergelt S (2010) Tracing mechanisms controlling the release of dissolved silicon in forest soil solutions using Si isotopes and Ge/Si ratios. Geochim Cosmochim Acta 74(14):3913–3924
Cornelis J-T, Titeux H, Ranger J, Delvaux B (2011) Identification and distribution of the readily soluble silicon pool in a temperate forest soil below three distinct tree species. Plant Soil 342(1):369–378
De Tombeur F, Turner B, Laliberté E, Lambers H, Mahy G, Faucon M-P, Zemunik G, Cornelis J-T (2020) Plants sustain the terrestrial silicon cycle during ecosystem retrogression. Science 369(6508):1245–1248
Delvigne C, Opfergelt S, Cardinal D, Delvaux B, André L (2009) Distinct silicon and germanium pathways in the soil-plant system: Evidence from banana and horsetail. J Geophys Res 114(2):124–131. https://doi.org/10.1029/2008jg000899
Derry LA, Kurtz AC, Ziegler K, Chadwick OA (2005) Biological control of terrestrial silica cycling and export fluxes to watersheds. Nature 433(7027):728–731
Epstein E (1994) The anomaly of silicon in plant biology. Proc Natl Acad Sci USA 91(1):11–17
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281(5374):237–240
Fraysse F, Cantais F, Pokrovsky OS, Schott J, Meunier J-D (2006) Aqueous reactivity of phytoliths and plant litter: physico-chemical constraints on terrestrial biogeochemical cycle of silicon. J Geochem Explor 88(1–3):202–205
Froelich P, Blanc V, Mortlock R, Chillrud S, Dunstan W, Udomkit A, Peng TH (1992) River fluxes of dissolved silica to the ocean were higher during glacials: Ge/Si in diatoms, rivers, and oceans. Paleoceanography 7(6):739–767
Fulweiler RW, Nixon SW (2005) Terrestrial vegetation and the seasonal cycleof dissolved silica in a southern New Englandcoastal river. Biogeochemistry 74(1):115–130
Gérard F, Mayer K, Hodson M, Ranger J (2008) Modelling the biogeochemical cycle of silicon in soils: application to a temperate forest ecosystem. Geochim Cosmochim Acta 72(3):741–758
Giblin AE, Tobias CR, Song B, Weston N, Banta GT, H. RIVERA-MONROY V (2013) The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 26(3):124–131
Giblin AE, Howarth RW (1984) Porewater evidence for a dynamic sedimentary iron cycle in salt marshes 1. Limnol Oceanogr 29(1):47–63
Goñi MA, Gardner IR (2003) Seasonal dynamics in dissolved organic carbon concentrations in a coastal water-table aquifer at the forest-marsh interface. Aquat Geochem 9(3):209–232
Gonneea ME, Charette MA (2014) Hydrologic controls on nutrient cycling in an unconfined coastal aquifer. Environ Sci Technol 48(24):14178–14185
Gonneea ME, Maio CV, Kroeger KD, Hawkes AD, Mora J, Sullivan R, Madsen S, Buzard RM, Cahill N, Donnelly JP (2019) Salt marsh ecosystem restructuring enhances elevation resilience and carbon storage during accelerating relative sea-level rise. Estuar Coast Shelf Sci 217:56–68
Granum E, Raven JA, Leegood RC (2005) How do marine diatoms fix 10 billion tonnes of inorganic carbon per year? Can J Bot 83(7):898–908
Granville KE, Ooi SK, Koenig LE, Lawrence BA, Elphick CS, Helton AM (2021) Seasonal patterns of denitrification and N2O production in a southern New England salt marsh. Wetlands 41(1):1–13
Hodson M, White P, Mead A, Broadley M (2005) Phylogenetic variation in the silicon composition of plants. Ann Bot 96(6):1027–1046
Hodson MJ, Evans DE (2020) Aluminium–silicon interactions in higher plants: an update. J Exp Bot 71(21):6719–6729
Howes BL, Goehringer DD (1994) Porewater drainage and dissolved organic carbon and nutrient losses through the intertidal creekbanks of a New England salt marsh. Mar Ecol Prog Ser Oldendorf 114(3):289–301
Irigoien X, Harris RP, Verheye HM, Joly P, Runge J, Starr M, Pond D, Campbell R, Shreeve R, Ward P (2002) Copepod hatching success in marine ecosystems with high diatom concentrations. Nature 419(6905):387–389
Kaplan W, Valiela I, Teal JM (1979) Denitrification in a salt marsh ecosystem 1. Limnol Oceanogr 24(4):726–734
Kearney MS, Turner RE (2016) Microtidal marshes: can these widespread and fragile marshes survive increasing climate–sea level variability and human action? J Coast Res 32(3):686–699
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47(260):583–621
Kurtz AC, Derry LA, Chadwick OA (2002) Germanium-silicon fractionation in the weathering environment. Geochim Cosmochim Acta 66(9):1525–1537
Kurtz AC, Lugolobi F, Salvucci G (2011) Germanium-silicon as a flow path tracer: application to the Rio Icacos watershed. Water Resour Res. https://doi.org/10.1029/2010WR009853
Lancelot C (1995) The mucilage phenomenon in the continental coastal waters of the North Sea. Sci Total Environ 165(1–3):83–102
Leblanc K, Leynaert A, Rimmelin P, Moutin T, Raimbault P, Ras J, Quéguiner B (2005) A seasonal study of diatom dynamics in the North Atlantic during the POMME experiment (2001): evidence for Si limitation of the spring bloom. J Geophys Res. https://doi.org/10.1029/2004JC002621
Lugolobi F, Kurtz AC, Derry LA (2010) Germanium–silicon fractionation in a tropical, granitic weathering environment. Geochim Cosmochim Acta 74(4):1294–1308
Luther GW, Church TM, Scudlark JR, Cosman M (1986) Inorganic and organic sulfur cycling in salt-marsh pore waters. Science 232(4751):746–749
Luther GW III, Giblin A, Howarth RW, Ryans RA (1982) Pyrite and oxidized iron mineral phases formed from pyrite oxidation in salt marsh and estuarine sediments. Geochim Cosmochim Acta 46(12):2665–2669
Mann AG, Gonneea JA, Brosnahan ME, Brooks SM, Wang TW, Ganju ZA, Kroeger NK, Kevin D (2019) Time-series of biogeochemical and flow data from a tidal salt-marsh creek. Sage Lot Pond, Waquoit Bay, Massachusetts (2012–2016)
Mann HB, Whitney DR (1947) On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat 18(1):50–60
MATLAB (2021b) The MathWorks Inc., Natick, Massachusetts
Mitani N, Ma JF (2005) Uptake system of silicon in different plant species. J Exp Bot 56(414):1255–1261
Mortlock RA, Frohlich PN (1987) Continental weathering of germanium: GeSi in the global river discharge. Geochim Cosmochim Acta 51(8):2075–2082
Moseman-Valtierra S, Abdul-Aziz OI, Tang J, Ishtiaq KS, Morkeski K, Mora J, Quinn RK, Martin RM, Egan K, Brannon EQ (2016) Carbon dioxide fluxes reflect plant zonation and belowground biomass in a coastal marsh. Ecosphere 7(11):e01560
Müller F, Struyf E, Hartmann J, Weiss A, Jensen K (2013) Impact of grazing management on silica export dynamics of Wadden Sea saltmarshes. Estuar Coast Shelf Sci 127:1–11
Murnane RJ, Stallard RF (1990) Germanium and silicon in rivers of the Orinoco drainage basin. Nature 344(6268):749–752
Nawaz MA, Zakharenko AM, Zemchenko IV, Haider MS, Ali MA, Imtiaz M, Chung G, Tsatsakis A, Sun S, Golokhvast KS (2019) Phytolith formation in plants: from soil to cell. Plants 8(8):249
NOAA National Estuarine Research Reserve System (NERRS) (2019) System-wide Monitoring Program. Data accessed from the NOAA NERRS Centralized Data Management Office website: http://www.nerrsdata.org. Accessed July 2019
Norris A, Hackney C (1999) Silica content of a mesohaline tidal marsh in North Carolina. Estuar Coast Shelf Sci 49(4):597–605
Officer C, Ryther J (1980) The possible importance of silicon in marine eutrophication. Mar Ecol Prog Ser 3(1):83–91
Perry DC, Chaffee C, Wigand C, Thornber C (2020) Implementing adaptive management into a climate change adaptation strategy for a drowning New England salt marsh. J Environ Manag 270:110928
Rahman S, Tamborski JJ, Charette MA, Cochran JK (2019) Dissolved silica in the subterranean estuary and the impact of submarine groundwater discharge on the global marine silica budget. Mar Chem 208:29–42
Raven JA (1983) The transport and function of silicon in plants. Biol Rev 58(2):179–207
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org. Accessed Jan 2019
Reed DJ, De Luca N, Foote AL (1997) Effect of hydrologic management on marsh surface sediment deposition in coastal Louisiana. Estuaries 20(2):301–311
Roberts K, Granum E, Leegood RC, Raven JA (2007) Carbon acquisition by diatoms. Photosynth Res 93(1):79–88
Rousseaux CS, Gregg WW (2014) Interannual variation in phytoplankton primary production at a global scale. Remote Sens 6(1):1–19
Ryves DB, Battarbee RW, Juggins S, Fritz SC, Anderson NJ (2006) Physical and chemical predictors of diatom dissolution in freshwater and saline lake sediments in North America and West Greenland. Limnol Oceanogr 51(3):1355–1368
Santos IR, Chen X, Lecher AL, Sawyer AH, Moosdorf N, Rodellas V, Tamborski J, Cho HM, Dimova N, Sugimoto R, Bonaglia S (2021) Submarine groundwater discharge impacts on coastal nutrient biogeochemistry. Nat Rev Earth Environ 2(5):307–323
Schaller J, Hodson MJ, Struyf E (2017) Is relative Si/Ca availability crucial to the performance of grassland ecosystems? Ecosphere 8(3):e01726
Smayda TJ (1990) Novel and nuisance phytoplankton blooms in the sea: evidence for a global epidemic. RWS-North Sea Directorate
Smayda TJ (1998) Patterns of variability characterizing marine phytoplankton, with examples from Narragansett Bay. ICES J Mar Sci 55(4):562–573
Smith KEL, Terrano JF, Khan NS, Smith CG, Pitchford JL (2021) Lateral shoreline erosion and shore-proximal sediment deposition on a coastal marsh from seasonal, storm, and decadal measurements. Geomorphology 389:107829
Song S, Wang ZA, Gonneea ME, Kroeger KD, Chu SN, Li D, Liang H (2020) An important biogeochemical link between organic and inorganic carbon cycling: effects of organic alkalinity on carbonate chemistry in coastal waters influenced by intertidal salt marshes. Geochim Cosmochim Acta 275:123–139
Street-Perrott FA, Barker PA (2008) Biogenic silica: a neglected component of the coupled global continental biogeochemical cycles of carbon and silicon. Earth Surf Process 33(9):1436–1457
Strickland J, Parsons T (1968) A practical handbook of seawater analysis. Bull Fish Res Bd Can 167:311
Struyf E, Conley DJ (2009) Silica: an essential nutrient in wetland biogeochemistry. Front Ecol Environ 7(2):88–94
Struyf E, Conley DJ (2012) Emerging understanding of the ecosystem silica filter. Biogeochemistry 107(1):9–18
Struyf E, Damme SV, Gribsholt B, Meire P (2005a) Freshwater marshes as dissolved silica recyclers in an estuarine environment. Hydrobiologia 540(1):69–77
Struyf E, Dausse A, Van Damme S, Bal K, Gribsholt B, Boschker HT, Middelburg JJ, Meire P (2006) Tidal marshes and biogenic silica recycling at the land-sea interface. Limnol Oceanogr 51(2):838–846
Struyf E, Smis A, Van Damme S, Meire P, Conley DJ (2009) The global biogeochemical silicon cycle. Silicon 1(4):207–213
Struyf E, Temmerman S, Meire P (2007) Dynamics of biogenic Si in freshwater tidal marshes: Si regeneration and retention in marsh sediments (Scheldt estuary). Biogeochemistry 82(1):41–53
Struyf E, Van Damme S, Gribsholt B, Middelburg JJ, Meire P (2005b) Biogenic silica in tidal freshwater marsh sediments and vegetation (Schelde estuary, Belgium). Mar Ecol Prog Ser 303:51–60
Tamborski JJ, Eagle M, Kurylyk BL, Kroeger KD, Wang ZA, Henderson P, Charette MA (2021) Pore water exchange-driven inorganic carbon export from intertidal salt marshes. Limnol Oceanogr 66(5):1774–1792
Tréguer PJ, De La Rocha CL (2013) The world ocean silica cycle. Ann Rev Mar Sci 5:477–501
Valiela I, Geist M, McClelland J, Tomasky G (2000) Nitrogen loading from watersheds to estuaries: verification of the Waquoit Bay nitrogen loading model. Biogeochemistry 49(3):277–293
Valiela I, McClelland J, Hauxwell J, Behr PJ, Hersh D, Foreman K (1997) Macroalgal blooms in shallow estuaries: controls and ecophysiological and ecosystem consequences. Limnol Oceanogr 42(5part2):1105–1118
Valiela I, Teal JM (1979) The nitrogen budget of a salt marsh ecosystem. Nature 280(5724):652–656
Vieillard AM, Fulweiler RW, Hughes ZJ, Carey JC (2011) The ebb and flood of silica: quantifying dissolved and biogenic silica fluxes from a temperate salt marsh. Estuar Coast Shelf Sci 95(4):415–423
Wang ZA, Kroeger KD, Ganju NK, Gonneea ME, Chu SN (2016) Intertidal salt marshes as an important source of inorganic carbon to the coastal ocean. Limnol Oceanogr 61(5):1916–1931
Watson E, Raposa K, Carey J, Wigand C, Warren R (2017) Anthropocene survival of southern New England’s salt marshes. Estuaries Coasts 40(3):617–625
West AJ, Galy A, Bickle M (2005) Tectonic and climatic controls on silicate weathering. Earth Planet Sci Lett 235(1–2):211–228
Weston NB (2014) Declining sediments and rising seas: an unfortunate convergence for tidal wetlands. Estuaries Coasts 37(1):1–23
White AF, Blum AE, Bullen TD, Vivit DV, Schulz M, Fitzpatrick J (1999) The effect of temperature on experimental and natural chemical weathering rates of granitoid rocks. Geochim Cosmochim Acta 63(19–20):3277–3291
White AF, Brantley SL (2003) The effect of time on the weathering of silicate minerals: why do weathering rates differ in the laboratory and field? Chem Geol 202(3–4):479–506
Wigand C, Ardito T, Chaffee C, Ferguson W, Paton S, Raposa K, Vandemoer C, Watson E (2017) A climate change adaptation strategy for management of coastal marsh systems. Estuaries Coasts 40(3):682–693
Yacano MR, Foster SQ, Ray NE, Oczkowski A, Raven JA, Fulweiler RW (2021) Marine macroalgae are an overlooked sink of silicon in coastal systems. The New phytologist:
Yamada SS, D’Elia CF (1984) Silicic acid regeneration from estuarine sediment cores. Mar Ecol Prog Ser Oldendorf 18(1):113–118
Acknowledgments
The authors thank U.S. Geological Survey staff including T. Wallace Brooks, Jennifer O’Keefe Suttles, and Adrian Mann for field and analytical efforts, and Jordan Mora and other staff at the Waquoit Bay National Estuarine Research Reserve for sampling support. Thanks to Thomas Ireland and James Baldwin for their mentorship and assistance with sample analysis and statistical analysis, respectively. We thank Kathryn Smith and two anonymous reviewers for their critical reviews of this manuscript and Christo Buizert for his feedback on an earlier draft.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection was performed by Meagan Eagle. Data analysis was performed by Olivia Williams, Joanna Carey, Meagan Eagle, and Joseph Tamborski. The first draft of the manuscript was written by Olivia Williams and all authors commented on and contributed to subsequent versions of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. This study was supported by the U.S. Geological Survey (USGS) Coastal and Marine Geology Program with support from the USGS Land Change Science Program’s LandCarbon program, U.S. National Science Foundation (OCE-1459521), NOAA Science Collaborative (NA09NOS4190153). Additional support was provided by ARCS Oregon, the Boston University (BU) Undergraduate Research Opportunities Program, and the BU Department of Earth and Environment. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Additional information
Responsible Editor: Justin B. Richardson
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Williams, O.L., Kurtz, A.C., Eagle, M.J. et al. Mechanisms and magnitude of dissolved silica release from a New England salt marsh. Biogeochemistry 161, 251–271 (2022). https://doi.org/10.1007/s10533-022-00976-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-022-00976-y