Abstract
Chromolaena odorata is one of the most common invasive plants, as the phosphorus input from guano in the coral islands continuously decreasing, causing substantial harm to the native vegetation in recent years. In the current study, we investigated the effects of soil phosphorous content, light intensity and competition on several physiological traits (plant height, leaf area, maximum net photosynthetic rate, and relative growth rate) of C. odorata and the native species Pisonia grandis and Scaevola taccada based on a greenhouse experiment with two light intensities and three levels of soil available phosphorus (P) content. We also evaluated the effects of light intensity and soil phosphorus content (and their interaction) on the relative yield and aggressivity coefficient of the invasive species C. odorata. The results showed that low light intensity significantly inhibited the growth of the three species. However, the high P treatment significantly inhibited the growth of C. odorata and P. grandis and significantly increased the growth of S. taccada under full-light conditions. The effect of soil P content on the interspecific competition among C. odorata, P. grandis, and S. taccada was mediated by light intensity and species combination. The high P treatment significantly reduced the competitive advantage of C. odorata over P. grandis. The results demonstrate that shaded habitats with a high soil P content could restrict invasion by C. odorata. This suggests that the invasiveness of C. odorata in tropical coral islands can be reduced by protecting native vegetation and thus increasing shade and soil P content.
Similar content being viewed by others
Introduction
Alien plant invasion results in severe socioeconomic and environmental damage worldwide (Rani et al. 2020; Vilà et al. 2011). Preventing plant invasion is a research hotspot in ecology and has attracted worldwide attention (Bradley et al. 2012; Hulme et al. 2018). Island ecosystems are highly damaged by invasive plant species (Fenouillas et al. 2021; Greimler et al. 2002). The negative effects of invasive plants on islands are worse than those on the mainland (Pyšek et al. 2012). Moreover, the destruction of native vegetation due to plant invasion will further affect indigenous animals and microorganisms and reduce biodiversity (Cai et al. 2020; Sax and Gaines 2008).
Successful invasion by exotic plant species depends on many factors, including light intensity and soil nutrient levels (Daehler 2003). For example, Chen et al. (2020) found that increased soil phosphorus (P) content significantly reduced the competitive advantage of the invasive species Bidens pilosa and Eupatorium catarium. In addition, soil nutrients, including N content and use efficiency and the N/P ratio, affect the growth of invasive and native plants (Geng and He 2021; Yu and He 2021; Yuan 2014). Differences in the responses of invasive and native species to low light intensity are another important factor affecting the successful establishment and expansion of invasive plants (Feng et al. 2007; Martin et al. 2009). Paudel and Battaglia (2021) reported that some invasive plants were more shade tolerant than native plants in the coastal region of the southeastern USA. Although the relationship between invasiveness and the plasticity of physiological traits in response to irradiance is complex (Zheng et al. 2012), low light intensity reduces the invasiveness of Sonneratia apetala (Chen et al. 2013). However, the factors contributing to plant invasion vary across ecosystems, especially in areas with high soil available phosphorus content and that are far from the mainland, such as tropical coral islands (Cai et al. 2020).
The Paracel Islands in the South China Sea are a group of tropical coral islands that are formed mostly by coral sands, guano, and plant residues (Xu et al. 2011). The understory soil is strongly alkaline with a pH range of 8.0–9.5, and it is rich in phosphorus and calcium but lacks iron and aluminum (Jian 2020). Restricted by sea tides, typhoons, and limited availability of soil nutrients, only a few natural plant species can live in there. The vegetation is characterized by the dominant native shrub species Scaevola taccada and the tree species Pisonia grandis (Huang et al. 2021; Jian 2020). However, the Paracel Islands have also faced a serious threat of plant invasion in recent years, and there have been relatively few studies on the effects of plant invasion on these islands (Cai et al. 2020). The majority of plant communities in tropical islands could be dominated by invasive species, preventing subsequent recolonization by native plants (Huang et al. 2021). It is important to protect native plant communities and prevent and control plant invasions to support biodiversity conservation and the sustainability of island ecosystems.
Chromolaena odorata (L.) R.M. King & H. Rob., a perennial plant that is native to Central and South America, has invaded many ecosystems worldwide (Yu et al. 2010). In 1934, C. odorata was first found in mountainous areas in Yunnan and Hainan provinces, China; it then quickly spread and threat ecosystems in South China (Xu et al. 2020). Chromolaena odorata is currently one of the main invasive plants in the Paracel Islands (Cai et al. 2020) (Fig. 1a). In addition, studies have found that C. odorata has a rapid growth rate and strong reproductive ability (Yu et al. 2014), inhibits the growth of native plants and causes serious damage to tropical island ecosystems (Cai et al. 2020). Shi et al. (2020) found that C. odorata has higher irradiance utilization efficiency than native plants and strong photosynthetic resistance to damage caused by irradiance. These traits may facilitate C. odorata invasion of native plant communities. However, little is known about how to prevent and control invasions in tropical islands by C. odorata. Our previous investigation found that the successful invasion of C. odorata might be associated with the decrease in soil P in the Paracel Islands (Cai et al. 2020). Therefore, it is necessary to expand on previous knowledge by investigating competition for light and nutrients by C. odorata in tropical coral islands.
Pisonia grandis R. Brown is a native and widespread tree species on atolls and islands that are heavily influenced by guano additions (Hayward and Horton 2012) and is now the most common native tree species in the Paracel Islands (Fig. 1a). Scaevola taccada (Gaertner) Roxb. is a native and dominant shrub species in coastal strand plant communities (Goldstein et al. 1996) and is the most common native shrub species in the Paracel Islands (Fig. 1a). P. grandis grows in the centers of the islands where the soil P content is highest, and S. taccada grows closer to the coastline where the P content is relatively low. Protecting these two native species is important to the ecology and socioeconomic status of the Paracel Islands (Wang et al. 2019).
The establishment of plant communities in the Paracel Islands is closely related to the activities of seabirds, who deposit large quantities of P and other nutrients on the islands in their feces and thereby promote the development of phospho-calcitic soils (Gong et al. 2013). In addition, native vegetation provides shelters and breeding space for seabirds. Unfortunately, the number of seabirds in the Paracel Islands has decreased due to anthropogenic disturbance (Cao et al. 2007), and this decrease has greatly reduced the input of guano-derived organic matter to the islands (Wu et al. 2017). In addition, anthropogenic disturbance has severely altered the original ecosystems and thereby increased the possibility of invasion (Rodgers III and Parker 2003). However, no study has yet assessed the effects of guano reduction on biological invasions.
In this study, we conducted a greenhouse experiment with different light intensities and soil available phosphorus contents (P), aiming to (1) determine the effects of soil phosphorus content, light intensity, and competition on several growth and physiological traits of the invasive species C. odorata and the native species P. grandis and S. taccada and (2) evaluate the effects of light intensity and soil phosphorus content on the relative yield and aggressivity coefficient of the invasive species C. odorata.
Materials and methods
Soil and plant materials
The experiment was conducted in a greenhouse at the South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, China (23° 10′ N, 113° 21′ E).
The seeds of the native species P. grandis and S. taccada and the invasive species C. odorata were collected from Yongxing Island (16° 49′ N, 112° 20′ E) in the Paracel Islands in December 2019. We picked fully ripe fruits from several individuals per species and immediately collected mature seeds. Then, the mature seeds were separately stored in zip-lock bags in a refrigerator at 4 °C for subsequent use.
To simulate the soil conditions of the Paracel Islands, we collected soil from the 0–20 cm soil layer from the native communities of P. grandis and S. taccada in the Paracel Islands. The soils were mixed evenly at a mass ratio of 1:1 and then mixed with coral sand at a soil to sand mass ratio of 1:5. The mean contents of total P, available P, and total nitrogen (N) in the final soil were measured as described by Liu et al. (1996), with values of 12.08 g kg−1, 53.89 mg kg−1, and 4.26 g kg−1, respectively. The soil was placed in pots (18 cm bottom inner diameter, 32 cm top inner diameter, and 21 cm height). Because the seedlings differed in germination and growth rates, we sowed the seeds of P. grandis and S. taccada on January 1, 2020, and the seeds of C. odorata on June 1, 2020. The seeds were separately sown in germination trays in a greenhouse with an average air temperature of 28 °C and humidity of 65%.
Experimental conditions
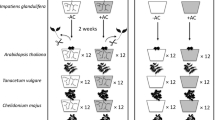
To simulate the conditions in the Paracel Islands, we estimated the mean light intensity under the native plant communities to be about 10% of that in the open land of the original community of understory plants. In addition, we measured soil available P at different locations in the Paracel Islands and found soil available P contents ranging from 53.89 mg kg−1 − 1053.89 mg kg−1. Therefore, we conducted the experiment with two light intensities (full light and 10% light) and three levels of available P: low P (53.89 mg kg−1), medium P (253.89 mg kg−1), and high P (1053.89 mg kg−1). A sunshade net that blocked 90% of the light was used to obtain 10% light. NaH2PO4·2H2O (Analytical Pure, Guangzhou Chemical Reagent Factory) was used to obtain the three levels of available P (Li et al. 2019).
On June 25, 2020, we selected seedlings of each species with the same numbers of green leaves and buds and similar heights and basal diameters. The P. grandis and S. taccada seedlings were approximately 8 cm (7–9 cm) tall, and the C. odorata seedlings were approximately 5 cm (4.5–5.5 cm) tall before transplantation into pots. Then, we transplanted the seedlings into pots in five polyculture patterns: two C. odorata seedlings per pot (CC); two P. grandis seedlings per pot (PP); two S. taccada seedlings per pot (SS); one C. odorata seedling and one P. grandis seedling per pot (CP); and one C. odorata seedling and one S. taccada seedling per pot (CS).
After 2 weeks of acclimatization in the greenhouse, the seedlings were divided into two groups for the full light and 10% light treatments. The pots were placed on flat well-drained soil with 20 cm between adjacent pots. Half the area occupied by the pots was covered with the sunshade net (at 200 cm above the soil surface). An LI-6800 portable photosynthesis system (Li-Cor, Lincoln, NE, USA) was used for 3 consecutive days to define the full light treatment and the low light treatment at 9 am, 12 noon, and 3 pm (Fig. 1b).
The pots were fertilized with 500 mL of P-free Hoagland solution every month to prevent malnutrition; each liter of solution contained 945 mg Ca(NO3)2·4H2O, 607 mg KNO3, 20 mg NH4NO3, 493 mg MgSO4·7H2O, 40 mg [CH2N(CH2COONa)CH2COO]2Fe, 2.86 mg H3BO3, 2.13 mg MnSO4·7H2O, 0.22 mg ZeSO4·7H2O, 0.08 mg CuSO4·5H2O, and 0.02 mg H8MoN2O4 (Quan et al. 2014). Each of the 30 treatments (2 light levels, 3 P levels, and 5 plant combinations) was represented by 5 pots, for a total of 150 pots. F refers to full light intensity, and L refers to 10% light intensity; P1, P2, and P3 refer to low, medium, and high phosphorus levels, respectively; CC indicates two C. odorata seedlings per pot; PP indicates two P. grandis seedlings per pot; SS indicates two S. taccada seedlings per pot; CP indicates one C. odorata seedling interplanted with one P. grandis seedling; and CS indicates one C. odorata seedling interplanted with one S. taccada seedling (Fig. 1c).
Growth measurements
After 3 months, we measured the height of each plant and collected all the leaves from each plant. We then measured the area of each leaf using a portable leaf area meter (LI-3000 A, Li-Cor, Lincoln, NE, USA).
The roots in each pot were carefully removed under running water and then washed with water. The roots, stems, and leaves were then separately dried at 105 ℃ for 1 h followed by drying at 65 ℃ until they reached a constant dry weight.
At the beginning of the experiment (T0), the initial total biomass (W1) of the seedlings and their heights were determined. After 90 days (T1), the total biomass (W2) of each plant was measured. The following formula was used to calculate the relative growth rate (RGR) (Yamashita et al. 2002):
Photosynthesis measurement
Before plants were harvested as described in the previous section, an LI-6800 portable photosynthesis system equipped with a red/blue LED light source was used to determine the maximum net photosynthetic rate of healthy mature leaves at the top of each plant on clear days. All measurements were repeated three times with a small CO2 cylinder to ensure the stability of the airflow for each plant using different mature leaves. The measurements were carried out with a photo flux density of 1500 µmol m−² s−¹ and an ambient CO2 concentration of 400 µmol mol−1.
Relative yield and aggressivity coefficient
The mean total biomass of the invasive species and the native species were recorded for each treatment, and relative yield (RY) and the aggressivity coefficient (AG) were used to measure the competitiveness of the species. RY per plant was calculated for each species in each replicate using the following equation (Shipley and Keddy 1994):
RYc is the relative yield of C. odorata; RYn is the relative yield of a native species; Ycn is the biomass of a single C. odorata growing with a native species; Yc is the biomass of two C. odorata in monoculture; Ync is the biomass of a single plant of a native species growing with C. odorata; and Yn is the biomass of two plants of the native species growing in monoculture.
RY values < 1 indicate that interspecific competition was greater than intraspecific competition. RY values equal to 1 indicate that interspecific competition was equal to intraspecific competition. RY values > 1 indicate that intraspecific competition was greater than interspecific competition.
The aggressivity coefficient (AG) represented the competitive strength of C. odorata relative to that of the native plants and was calculated by the following equation (McGilchrist and Trenbath 1971):
AG values > 0 indicate that C. odorata was more competitive than the native plants. AG values equal to 0 indicate equal competitiveness between C. odorata and the native plants. AG values < 0 indicate that the native plants were more competitive than C. odorata.
Statistical analysis
Three-way analyses of variance (ANOVAs) were used to compare the effects of soil P content, light intensity, competition, and their interaction on plant height, leaf area, relative growth rate, and the maximum net photosynthesis rate. In addition, ANOVA and pairwise t tests with a Bonferroni correction were used to analyze the differences with light and interspecific competition treatments for each species under different soil P contents. The ggpubr and rstatix packages for R (x64) 4.1.2 were used for statistical analyses and creating graphics.
The effects of soil P content, light intensity, and their interaction on RY and AG were tested with two-way ANOVAs. Independent samples t tests were used to compare the differences between RY and 1 and between AG and 0. The differences in RY and AG among different P content treatments were analyzed by single factor analysis of variance combined with the least significant difference (LSD) test for post hoc determination. These statistical analyses were performed with the t.test, anova, and LSD.Test functions in R (x64) 4.1.2.
Results
Effects of P level, light intensity, and competition on plant height
The plant heights of C. odorata, S. taccada, and P. grandis under the full light intensity treatment were significantly higher than those under the low light intensity treatment (Fig. 2). Under the condition of full light intensity, the plant height of C. odorata in the medium P treatment was higher than that in the low P and high P treatments, and significant differences were detected between the medium P and high P treatments for C. odorata planted with P. grandis and between the medium P and low P treatments for C. odorata planted with S. taccada. These results show that soil P promotes the growth of C. odorata within a certain range of low to medium P levels but inhibits its growth when a threshold P level is exceeded, especially when it is planted with S. taccada or P. grandis. Interspecific competition between P. grandis and C. odorata led to significantly smaller plant heights of P. grandis than intraspecific competition among this species (Fig. 2). Under low light conditions without interspecific competition, the plant height of C. odorata was significantly higher in the high P treatment than in the low and medium P treatments. However, the plant height of C. odorata was not significantly different when planted with native species under low light regardless of the increase in P level. In addition, the plant height of S. taccada without interspecific competition was also significantly higher in the high P content treatment than in the medium P treatment under low light, but no statistically significant differences were observed for P. grandis (Fig. 2). Interestingly, the plant height of P. grandis was significantly greater in the high P treatment than in the low P treatment (Fig. 2).
Overall, low light intensity significantly reduced the heights of C. odorata (p < 0.01), S. taccada (p < 0.01), and P. grandis plants (p < 0.05). In addition, the P level and the interaction between light intensity and P level significantly affected the height of C. odorata (p < 0.05), while the stimulatory effect of high P was diminished by light and competition. In addition, the heights of P. grandis plants were also significantly affected by competition, and interspecific competition significantly reduced plant height (p < 0.01).
Effects of phosphorus level, light intensity, and competition on leaf area
When C. odorata was planted with S. taccada or P. grandis, the leaf area of C. odorata increased significantly, while that of S. taccada and P. grandis decreased significantly compared to monocultural planting (Fig. 3). Interestingly, the leaf area of C. odorata was inhibited in the high P significantly in full light conditions. In addition, the leaf area of P. grandis decreased significantly under the high P and full light treatment regardless of competition (Fig. 3). However, under low light intensity, the leaf area of P. grandis plants significantly increased as the content of soil available P increased (p < 0.01). There was no significant difference in S. taccada leaf area among P levels (Fig. 3).
Overall, the leaf areas of C. odorata, S. taccada, and P. grandis were significantly affected by light intensity and competition (p < 0.01). Low light reduced the leaf area of C. odorata and S. taccada while increasing the leaf area of P. grandis. However, the leaf area of C. odorata was increased when it was planted with native species, while the leaf areas of S. taccada and P. grandis were lower in the presence of interspecific competition than with no competition. In addition, the P level significantly affected the leaf area of C. odorata (p < 0.05), and high P reduced the leaf area under full light in the presence of competition with native species.
Effects of phosphorus level, light intensity, and competition on photosynthesis
To examine the effects of P content, light intensity, competition, and their interactions on photosynthesis, we measured the maximum net photosynthetic rate (MNPR) of mature leaves in the experimental plants. Our results showed that low light intensity significantly decreased the MNPR of C. odorata (p < 0.05) and S. taccada (p < 0.01) (Fig. 4), while that of P. grandis was unaffected. Interestingly, when C. odorata was planted with native plants, the MNPR of C. odorata significantly decreased under the high P content and full light intensity conditions (Fig. 4). In addition, in the treatment with full light and without interspecific competition, the MNPR of S. taccada was higher in the high P treatment than in the medium P treatment, while the MNPR of P. grandis was higher in the low P treatment than in the low and medium P treatments (Fig. 4). However, the opposite trend was observed when S. taccada and P. grandis competed with C. odorata. The MNPR of S. taccada was significantly decreased under high P content, while the MNPR of P. grandis was significantly increased under high P content (Fig. 4).
Light intensity, P level, and their interactions significantly affected the MNPR of C. odorata, and low light intensity caused the MNPR to decrease. Competition had slight effects on the maximum photosynthetic rate of C. odorata but had significant effects on that of S. taccada and P. grandis (p < 0.01). Additionally, the MNPR of S. taccada was significantly affected by light (p < 0.01), with the value being lower under low light than under full light.
Effects of phosphorus level, light intensity, and competition on relative growth rates
The relative growth rate (RGR) directly reflects the total biomass accumulation rate in a period and represents the competitive difference between invasive plants and native plants under different treatments. P level (p < 0.05), light intensity (p < 0.01) and the interaction of P level and light intensity (p < 0.05) significantly affected the RGR of C. odorata. The RGR of S. taccada was significantly affected by light (p < 0.01) and the interaction of light and competition (p < 0.05), and the RGR of P. grandis was significantly affected by light, competition, and their interactions (p < 0.05).
Among all the treatments, the light intensity had the largest impact on the RGRs of C. odorata, S. taccada, and P. grandis, and the RGRs of these plant species with full light intensity were significantly greater than those with low light intensity (p < 0.01) (Fig. 5). In addition, when C. odorata competed with native plants under full light intensity, the RGR of C. odorata significantly decreased under high P conditions compared to that under medium P conditions, but the RGR of P. grandis plants significantly increased under medium P conditions (Fig. 5). In addition, interspecific competition significantly decreased the RGR of P. grandis, although the high P level improved it significantly (Fig. 5). Under low light intensity, the RGR of P. grandis was significantly higher in the high P treatment than in the medium P or low P treatments, while the RGR of S. taccada showed no significant difference among the three P levels (Fig. 5).
Effects of phosphorus level, light intensity, and competition on relative yield and aggressivity coefficient
The relative competitiveness of P. grandis, S. taccada, and C. odorata was compared using the relative yield (RY) and the aggressivity coefficient (AG). Light intensity significantly affected the RY of C. odorata and S. taccada, and the P level significantly affected only the RY of C. odorata in competition with S. taccada (p < 0.05). The AG of C. odorata was significantly affected by light intensity and the interaction between light intensity and P level (p < 0.05) (Table 1). The RY of P. grandis was significantly affected by light intensity, P level, and their interaction, while the RY of C. odorata was significantly affected by only light intensity (p < 0.05). The AG of C. odorata was significantly affected by light intensity, P level, and competition with P. grandis (p < 0.05) (Table 1).
Under full light, the RY of C. odorata increased as the P level increased and was significantly greater at medium and high P levels than at the low P level (p < 0.05). However, the RY of S. taccada decreased with increasing P level and was significantly greater at the low P level than at the high P level (p < 0.05). The AG of C. odorata increased significantly as the P level increased, and it was significantly greater at high P levels than at medium or low P levels (p < 0.05). Interestingly, under low light, AG values < 0 indicated that S. taccada was more competitive than C. odorata (Table 2).
The RY of P. grandis was smallest with low P and full light, and it was significantly greater with high P than with medium or low P under low light (p < 0.05). However, the RY of C. odorata was the smallest with high P. The AG of C. odorata was significantly higher with high P than with low or medium P under full light (p < 0.05). With the increase in P level, the AG of C. odorata declined, indicating a reduction in competitiveness under full or low light (Table 2).
Overall, competition between C. odorata and P. grandis or S. taccada was affected by both light and P levels. Especially with low light, increases in the P content of the soil reduced the ability of C. odorata to compete with the native species.
Discussion
Low light intensity inhibits the invasion of C. odorata in tropical coral islands
The ability to capture, utilize, and tolerate light is important for the success of many invasive species over native species (Feng et al. 2007; Zheng et al. 2009). Previous studies found that the tolerance of many invasive plants to low light accelerated their invasion (Martin et al. 2009; Zavala et al. 2007). C. odorata is a heliophile and is limited by light intensity (Yu et al. 2010). In our study, low light intensity significantly reduced the heights, leaf areas, biomass values, and relative growth rates of C. odorata, P. grandis, and S. taccada.
Plant biomass and growth largely depend on the efficiency of light capture and utilization (Meekins and McCarthy 2000; Okada and Katoh 1998). Moreover, plant height and leaf area are considered the main traits that affect plant competition for light resources (Gorchov and Trisel 2003). Both C. odorata and S. taccada can acquire more light resources under full light than under low light; that is, their growth was greater under full light. In contrast, the growth of P. grandis was not significantly greater with full light than with low light. This finding shows that C. odorata and S. taccada are more sensitive to light than P. grandis. Consistent with the view that plant growth is often more affected by light intensity than by the levels of soil nutrients (Wei et al. 2017), we found that the growth of C. odorata, P. grandis, and S. taccada did not significantly vary among P levels when the light intensity was low. Invasive plants with low shade tolerance have difficulty invading communities with high canopy closure (Sharma et al. 2022). Leaf structural and physiological traits are crucial for the growth of C. odorata under high light conditions, and this species had a higher Pmax, photosynthetic nitrogen-use efficiency, and relative growth rate in bright conditions than in deep shade (Liao et al. 2021; Zhang and Wen 2009). In addition, the higher ratio of palisade/leaf thickness and net photosynthetic rate/palisade thickness may be two mechanisms by which C. odorata maintains greater photosynthetic performance through palisade regulation (Shi et al. 2020). Our results suggested that it was difficult for C. odorata to invade areas with shade from dense and deep vegetation in tropical islands.
High soil phosphorus levels reduce the competition of C. odorata with native plants
Nucleic acid synthesis and energy conversion in plants require P, and P levels in soil may greatly affect plant communities (Mo et al. 2023; Zhang et al. 2022). Our results indicate that a high P level in soil significantly reduces the growth of C. odorata, which is consistent with the results obtained for Cassytha filiformis and Wedelia biflora (Cai et al. 2020). Increases in the availability of soil P weakened the ability of invasive plants to compete with native plants (Brewer and Cralle 2003; Chen et al. 2020). In contrast, other studies have found that P addition improves the growth of C. odorata (Quan et al. 2014; Suding et al. 2004). These inconsistent conclusions about whether P addition promotes or inhibits invasion may be due to differences in the initial available P content in soils and the addition level. Suding et al. (2004) conducted an experiment on a prairie with clay loam and added 0.2 g/m2 phosphorus per year, and Quan et al. (2014) transplanted C. odorata into river sand with a maximum soil P level of 0.60 g kg−1. The soil P content in these two studies was lower than that in our study, indicating that it may not reach the threshold of inhibition. In contrast, the soil we used was from the Paracel Islands, and the initial P content in the soil was higher than the average level on the mainland (Zhao 2006).
The reduced competition of C. odorata with native plants due to high P may be attributed to the limited adaptation of C. odorata and the high tolerance of native plants to high soil available P content. Zhang et al. (2021) found that C. odorata acclimate to low soil available P by changing foliar P fractions and nutrient addition. In addition, the leaves of C. odorata can accumulate more P under conditions of P limitation (Hu et al. 2019). High-P habitats are suitable for only a few native, dominant plants in tropical coral islands (Huang 2020).
Our results challenged some general assumptions that eutrophication and high P availability favor invasive plants. C. odorata had a substantial competitive advantage over P. grandis, but this advantage decreased as soil P increased. In contrast, P addition to soil increased the competitive advantage of C. odorata over S. taccada under full light. This difference may result from the secretion of allelochemicals by C. odorata, which causes the photosynthetic rate of native plants to decrease (Zheng et al. 2015, 2018). Moreover, the invasive species showed a higher growth rate by using more resources at a low carbon cost to improve its competitive ability. C. odorata might have a higher P utilization efficiency than native plants and allocate more P to metabolic P for photosynthesis (Sun et al. 2022). Wan et al. (2018) also found that P addition reduced the competitive advantage of an invasive weed under different nutritional conditions. In other studies, the combined effects of P, N, and other soil nutrients affected the growth and distribution of plants (Ye et al. 2022; Zhang et al. 2017) and may also inhibit invasion by C. odorata. If the P content of the soil is low, it can be increased by attracting more seabirds to settle and by adding guano obtained from guano-rich areas or commercially available seabird guano (Young et al. 2011).
Conclusions: controlling C. odorata by adjusting the soil phosphorus content and light intensity of the understory
Light intensity and soil P content significantly affect plant growth and survival (Holste et al. 2011). The competitive advantage of C. odorata over native plants is weak when the native plants have formed a high canopy over soil with a high P content. It follows that the invasion of C. odorata in tropical coral islands may be restricted by luxuriant native plant communities and stable seabird populations. Once native communities, such as those with P. grandis or S. taccada, are damaged, C. odorata may quickly invade and replace native plants. Therefore, the protection of native plant communities, seabirds, and ecosystems is crucial for preventing invasion by C. odorata. Vegetation also provides a habitat for seabirds, and seabird guano is an important source of soil P in tropical islands. For partially degraded plant communities in tropical coral islands, we suggest that fast-growing native plants be planted as soon as possible to increase canopy closure and thereby minimize the probability of invasion by C. odorata.
In summary, the results of our study showed that both light intensity and soil available phosphorus content affected the invasive species C. odorata in competition with the native species S. taccada and P. grandis. Our results suggest that the invasion of C. odorata in coral islands may be controlled by enhancing the growth of native plant and seabird communities. This study provides a scientific basis for the subsequent ecological restoration and protection of local vegetation and ecosystems on coral islands.
Data availability
The data will be provided upon request on authors.
References
Brewer JS, Cralle SP (2003) Phosphorus addition reduces invasion of a longleaf pine savanna (Southeastern USA) by a non-indigenous grass (Imperata cylindrica). Plant Ecol 167:237–245. https://doi.org/10.1023/A:1023984214512
Bradley BA, Blumenthal DM, Early R, Grosholz ED, Lawler JJ, Miller LP, Sorte CJB, D’Antonio CM, Diez JM, Dukes JS, Ibanez I, Olden JD (2012) Global change, global trade, and the next wave of plant invasions. Front Ecol Environ 10:20–28. https://doi.org/10.1890/110145
Cao L, Pan YL, Liu NF (2007) Waterbirds of the Xisha Archipelago, South China Sea. Waterbirds 30:296–300. https://doi.org/10.1675/1524-4695(2007)30[296:WOTXAS]2.0.CO;2
Chen L, Peng S, Li J, Lin Z, Zeng Y (2013) Competitive control of an exotic mangrove species: restoration of native mangrove forests by altering light availability. Restor Ecol 21:215–223. https://doi.org/10.1111/j.1526-100X.2012.00892.x
Cai H, Lu H, Tian Y, Liu Z, Huang Y, Jian S (2020) Effects of invasive plants on the health of forest ecosystems on small tropical coral islands. Ecol Indic 117:106656. https://doi.org/10.1016/j.ecolind.2020.106656
Chen E, Liao H, Chen B, Peng S (2020) Arbuscular mycorrhizal fungi are a double-edged sword in plant invasion controlled by phosphorus concentration. New Phytol 226:295–300. https://doi.org/10.1111/nph.16359
Daehler CC (2003) Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu Rev Ecol Evol Syst 34:183–211. https://doi.org/10.1146/annurev.ecolsys.34.011802.132403
Feng Y, Wang J, Sang W (2007) Biomass allocation, morphology and photosynthesis of invasive and noninvasive exotic species grown at four irradiance levels. Acta Oecol 31:40–47. https://doi.org/10.1016/j.actao.2006.03.009
Fenouillas P, Ah-Peng C, Amy E et al (2021) Quantifying invasion degree by alien plants species in Reunion Island. Austral Ecol 46:1025–1037. https://doi.org/10.1111/aec.13048
Geng XM, He WM (2021) Success of native and invasive plant congeners depends on inorganic nitrogen compositions and levels. J Plant Ecol 14:202–212. https://doi.org/10.1093/jpe/rtaa088
Gorchov DL, Trisel DE (2003) Competitive effects of the invasive shrub, Lonicera maackii (Rupr.) Herder (Caprifoliaceae), on the growth and survival of native tree seedlings. Plant Ecol 166:13–24. https://doi.org/10.1023/A:1023208215796
Gong ZT, Zhang GL, Yang F (2013) Soils and the soil ecosystem in the South China Sea Islands. Ecol Environ Sci 22:183–188
Goldstein G, Drake DR, Alpha C, Melcher P, Heraux J, Azocar A (1996) Growth and photosynthetic responses of scaevola sericea, a hawaiian coastal shrub, to substrate salinity and salt spray. Int J Plant Sci 157:171–179. https://doi.org/10.1086/297336
Greimler J, Stuessy TF, Swenson U, Baeza CM, Matthei O (2002) Plant invasions on an Oceanic Archipelago. Biol Invasions 4:73–85. https://doi.org/10.1023/A:1020565510507
Huang Y (2020) Characteristics of soil seed bank of vegetations on tropical corals in Paracel Islands. Dissertation, University of Chinese Academy of Sciences (in Chinese)
Hayward JA, Horton TR (2012) Edaphic factors do not govern the ectomycorrhizal specificity of Pisonia grandis (Nyctaginaceae). Mycorrhiza 22:647–652. https://doi.org/10.1007/s00572-012-0442-2
Holste EK, Kobe RK, Vriesendorp CF (2011) Seedling growth responses to soil resources in the understory of a wet tropical forest. Ecology 92:1828–1838. https://doi.org/10.1890/10-1697.1
Hulme PE, Brundu G, Carboni M et al (2018) Integrating invasive species policies across ornamental horticulture supply chains to prevent plant invasions. J Appl Ecol 55:92–98. https://doi.org/10.1111/1365-2664.12953
Hu CC, Lei YB, Tan YH, Sun XC, Xu H, Liu CQ, Liu XY (2019) Plant nitrogen and phosphorus utilization under invasive pressure in a montane ecosystem of tropical China. J Ecol 107:372–386. https://doi.org/10.1111/1365-2745.13008
Huang Y, Ren H, Wang J, Liu N, Jian S, Cai H, Hui D, Guo Q (2021) Effects of Wollastonia biflora expansion on the soil seed bank in native forest communities on a tropical coral island. Glob Ecol Conserv 25:e01403. https://doi.org/10.1016/j.gecco.2020.e01403
Jian SG (2020) The vegetation in tropical coral islands of China. Guihaia 40:443 (in Chinese)
Liao MC, Liu N, Jian SG (2021) Ecophysiological adaptability of Chromolaena odorata to tropical coral islands. Guihaia 41:905–913 (in Chinese)
Liu GS, Jiang NH, Zhang LD et al (1996) Soil physical and chemical analysis and description of soil profiles. Standards Press of China, Beijing (in Chinese)
Li Y, Tian D, Wang J, Niu S, Tian J, Ha D, Qu Y, Jing G, Kang X, Song B (2019) Differential mechanisms underlying responses of soil bacterial and fungal communities to nitrogen and phosphorus inputs in a subtropical forest. PeerJ 7:e7631. https://doi.org/10.7717/peerj.7631
McGilchrist CA, Trenbath BR (1971) A revised analysis of plant competition experiments. Biometrics 27:659–671. https://doi.org/10.2307/2528603
Meekins JF, McCarthy BC (2000) Responses of the biennial forest herb Alliaria petiolata to variation in population density, nutrient addition and light availability. J Ecol 88:447–463. https://doi.org/10.1046/j.1365-2745.2000.00461.x
Martin PH, Canham CD, Marks PL (2009) Why forests appear resistant to exotic plant invasions: intentional introductions, stand dynamics, and the role of shade tolerance. Front Ecol Environ 7:142–149. https://doi.org/10.1890/070096
Mo S, He S, Sang Y, Li J, Kashif M, Zhang Z, Su G, Jiang C (2023) Integration of microbial transformation mechanism of polyphosphate accumulation and sulfur cycle in subtropical marine mangrove ecosystems with spartina alterniflora invasion. Microb Ecol 85:478–494. https://doi.org/10.1007/s00248-022-01979-w
Okada K, Katoh S (1998) Two long-term effects of light that control the stability of proteins related to photosynthesis during senescence of rice leaves. Plant Cell Physiol 39:394–404. https://doi.org/10.1093/oxfordjournals.pcp.a029382
Paudel S, Battaglia LL (2021) Linking responses of native and invasive plants to Hurricane disturbances: implications for coastal plant community structure. Plant Ecol 222:133–148. https://doi.org/10.1007/s11258-020-01093-2
Pyšek P, Jarošík V, Hulme PE, Pergl J, Hejda M, Schaffner U, Vilà M (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob Change Biol 18:1725–1737. https://doi.org/10.1111/j.1365-2486.2011.02636.x
Quan GM, Xie JF, Zhang JE, Mao DJ (2014) Effects of nitrogen and phosphorus nutrients on the nutritive organ phenotypic plasticity of invasive Chromolaena odorata. Chin J Ecol 33:2625–2632
Rodgers JC III, Parker KC (2003) Distribution of alien plant species in relation to human disturbance on the Georgia Sea Islands. Divers Distrib 9:385–398. https://doi.org/10.1046/j.1472-4642.2003.00036.x
Rani S, Ahmed MK, Xiongzhi X, Yuhuan J, Keliang C, Islam MM (2020) Economic valuation and conservation, restoration and management strategies of Saint Martin’s coral island, Bangladesh. Ocean Coast Manag 183:105024. https://doi.org/10.1016/j.ocecoaman.2019.105024
Shipley B, Keddy PA (1994) Evaluating the evidence for competitive hierarchies in plant communities. Oikos 69:340–345. https://doi.org/10.2307/3546158
Sax DF, Gaines SD (2008) Species invasions and extinction: the future of native biodiversity on islands. Proc Natl Acad Sci USA 105(Suppl 1):11490–11497. https://doi.org/10.1073/pnas.0802290105
Shi X, Liu G, Zheng YL (2020) Comparisons of irradiance utilization efficiency by invasive Chromolaena odorata and its three co-occurring species in one planted understory. Flora 271:151680. https://doi.org/10.1016/j.flora.2020.151680
Suding KN, LeJeune KD, Seastedt TR (2004) Competitive impacts and responses of an invasive weed: dependencies on nitrogen and phosphorus availability. Oecologia 141:526–535. https://doi.org/10.1007/s00442-004-1678-0
Sharma LN, Adhikari B, Watson MF, Shrestha BB, Paudel E, Karna B, Rijal DP (2022) Forest canopy resists plant invasions: a case study of Chromolaena odorata in Sal (Shorea robusta) forests of Nepal. J Trop Ecol 38:49–57. https://doi.org/10.1017/S0266467421000456
Sun F, Zeng L, Cai M et al (2022) An invasive and native plant differ in their effects on the soil food-web and plant-soil phosphorus cycle. Geoderma 410:115672. https://doi.org/10.1016/j.geoderma.2021.115672
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708. https://doi.org/10.1111/j.1461-0248.2011.01628.x
Wan LY, Qi SS, Zou CB, Dai ZC, Zhu B, Song YG, Du DL (2018) Phosphorus addition reduces the competitive ability of the invasive weed Solidago canadensis under high nitrogen conditions. Flora 240:68–75. https://doi.org/10.1016/j.flora.2017.12.012
Wu L, Liu X, Xu L (2017) Change of organic δ13C in ornithogenic sediments of the Xisha Archipelago, South China sea and its implication for ecological development. Acta Geol Sin-Engl Ed 91:1109–1119. https://doi.org/10.1111/1755-6724.13327
Wei H, Yan W, Quan G, Zhang J, Liang K (2017) Soil microbial carbon utilization, enzyme activities and nutrient availability responses to Bidens pilosa and a non-invasive congener under different irradiances. Sci Rep 7:11309. https://doi.org/10.1038/s41598-017-11707-x
Wang SH, Zhu YJ, Wang YF, Mao P, Liu N, Jian SG, Zhu LW, Liu H, Zhang W (2019) Effect of vegetation types on soil physicochemical property in East Island and Yongxing Island of Xisha Islands. J Trop Subtrop Bot 27:383–390
Xu LQ, Liu XD, Sun LG, Yan H, Liu Y, Luo YH, Huang J (2011) Geochemical evidence for the development of coral island ecosystem in the Xisha Archipelago of South China Sea from four ornithogenic sediment profiles. Chem Geol 286:135–145. https://doi.org/10.1016/j.chemgeo.2011.04.015
Xu QY, Wang D, Quan GM, Xiang HM, Zhang JE (2020) Invasive Chromolaena odorata species specifically affects growth of its co-occurring weeds. Ann N Y Acad Sci 1470:57–66. https://doi.org/10.1111/nyas.14330
Yuan Y (2014) Effects of different N:P ratio on interaction between the exotic plant Rhus typhina and the native plant Quercus acutissima and vitex negundo var. heterophylla. University of Shandong, Jinan
Yu HW, He WM (2021) Congeneric invasive versus native plants utilize similar inorganic nitrogen forms but have disparate use efficiencies. J Plant Ecol 14:180–190. https://doi.org/10.1093/jpe/rtaa085
Yamashita N, Koike N, Ishida A (2002) Leaf ontogenetic dependence of light acclimation in invasive and native subtropical trees of different successional status. Plant Cell Environ 25:1341–1356. https://doi.org/10.1046/j.1365-3040.2002.00907.x
Yu XQ, Feng YL, Li QM (2010) Review of research advances and prospects of invasive Chromolaena odorata. Chin J Plant Ecol 34:591–600. https://doi.org/10.3773/j.issn.1005-264x.2010.05.013
Young HS, McCauley DJ, Dirzo R (2011) Differential responses to guano fertilization among tropical tree species with varying functional traits. Am J Bot 98:207–214. https://doi.org/10.3732/ajb.1000159
Ye XQ, Ma RX, Lei ST, Wu M, Yu FH (2022) Maintained nutrient accumulation in invasive Solidago canadensis in response to competition: competition tolerance and nutrient accumulation. Flora 295:152136. https://doi.org/10.1016/j.flora.2022.152136
Yu X, He T, Zhao J, Li Q (2014) Invasion genetics of Chromolaena odorata (Asteraceae): extremely low diversity across Asia. Biol Invasions 16:2351–2366. https://doi.org/10.1007/s10530-014-0669-2
Zhao SP (2006) Evolution of seabird eco-environment on Xisha Islands of South China. Dissertation, University of Science and Technology of China (in Chinese)
Zhang LL, Wen DZ (2009) Structural and physiological responses of two invasive weeds, Mikania micrantha and Chromolaena odorata, to contrasting light and soil water conditions. J Plant Res 122:69–79. https://doi.org/10.1007/s10265-008-0197-1
Zavala MA, Angulo Ó, de la Parra RB, López-Marcos JC (2007) An analytical model of stand dynamics as a function of tree growth, mortality and recruitment: the shade tolerance-stand structure hypothesis revisited. J Theor Biol 244:440–450. https://doi.org/10.1016/j.jtbi.2006.08.024
Zheng YL, Feng YL, Liu WX, Liao ZY (2009) Growth, biomass allocation, morphology, and photosynthesis of invasive Eupatorium adenophorum and its native congeners grown at four irradiances. Plant Ecol 203:263–271. https://doi.org/10.1007/s11258-008-9544-5
Zheng YL, Feng YL, Lei YB, Liao ZY (2012) Comparisons of plastic responses to irradiance and physiological traits by invasive Eupatorium adenophorum and its native congeners. J Plant Physiol 169:884–891. https://doi.org/10.1016/j.jplph.2012.02.011
Zheng YL, Feng YL, Zhang LK, Callaway RM, Valiente-Banuet A, Luo DQ, Liao ZY, Lei YB, Barclay GF, Silva-Pereyra C (2015) Integrating novel chemical weapons and evolutionarily increased competitive ability in success of a tropical invader. New Phytol 205:1350–1359. https://doi.org/10.1111/nph.13135
Zhang H, Chang R, Guo X, Liang X, Wang R, Liu J (2017) Shifts in growth and competitive dominance of the invasive plant Alternanthera philoxeroides under different nitrogen and phosphorus supply. Environ Exp Bot 135:118–125. https://doi.org/10.1016/j.envexpbot.2016.12.014
Zheng YL, Burns JH, Liao ZY, Li YP, Yang J, Chen YJ, Zhang J, Zheng YG (2018) Species composition, functional and phylogenetic distances correlate with success of invasive Chromolaena odorata in an experimental test. Ecol Lett 21:1211–1220. https://doi.org/10.1111/ele.13090
Zhang L, Luo X, Lambers H, Zhang G, Liu N, Zang X, Xiao M, Wen D (2021) Effects of elevated CO2 concentration and nitrogen addition on foliar phosphorus fractions of Mikania micranatha and Chromolaena odorata under low phosphorus availability. Physiol Plant 173:2068–2080. https://doi.org/10.1111/ppl.13555
Zhang Y, Leng Z, Wu Y, Jia H, Yan C, Wang X, Ren G, Wu G, Li J (2022) Interaction between nitrogen, phosphorus, and invasive alien plants. Sustainability 14:746. https://doi.org/10.3390/su14020746
Acknowledgements
We thank the reviewers for their exhaustive and comprehensive comments on our manuscript.
Funding
This study was supported by the Guangdong Science and Technology Program (2019B21201005) and the National Key R&D Program of China(2022YFC3103700).
Author information
Authors and Affiliations
Contributions
JSG, LHX, LZF, and LMC contributed to the study conception and design; LMC, HLP, CHY, ZWM, XZH, OYKT, and YWY collected the data; LMC, HLP, and LHX analyzed the data; HLP, LMC, and JSG wrote the manuscript. All authors commented on previous versions of the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, L., Liao, M., Liao, H. et al. High phosphorus availability and low light intensity reduce the competitive ability of the invasive plant Chromolaena odorata in tropical coral islands. Biol Invasions 26, 471–487 (2024). https://doi.org/10.1007/s10530-023-03186-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03186-1