Abstract
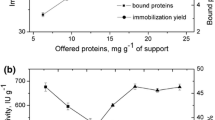
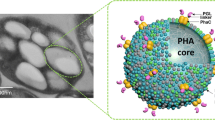
Polyhydroxyalkanoate (PHA) beads, recombinantly produced in Escherichia coli, were functionalized to display lipase B from Candida antarctica as translational protein fusion. The respective beads were characterized in respect to protein content, functionality, long term storage capacity and re-usability. The direct fusion of the PHA synthase, PhaC, to lipase B yielded active PHA lipase beads capable of hydrolyzing glycerol tributyrate. Lipase B beads showed stable activity over several weeks and re-usability without loss of function.


Similar content being viewed by others
References
Chen B, Hu J, Miller EM, Xie W, Cai M, Gross RA (2008) Candida antarctica lipase B chemically immobilized on epoxy-activated micro—and nanobeads: catalysts for polyester synthesis. Biomacromolecules 9:463–471
Goodman LP, Dugan LR Jr (1969) The effect of sonication on lipase activity. Lipids 5:362–365
Grage K, Jahns AC, Parlane N, Palanisamy R, Rasiah IA, Atwood JA, Rehm BHA (2009) Bacterial polyhydroxyalkanoate granules: biogenesis, structure, and potential use as nano-/micro-beads in biotechnological and biomedical applications. Biomacromolecules 10:660–669
Jaeger K-E, Reetz MT (1998) Microbial lipases form versatile tools for biotechnology. Trends Biotechnol 16:396–403
Jahns AC, Haverkamp RG, Rehm BHA (2008) Multifunctional inorganic-binding beads self-assembled inside engineered bacteria. Bioconjug Chem 19:2072–2080
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Ferndandez-Lafuente R (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol 40:1451–1463
Mei Y, Miller L, Gao W, Gross RA (2003) Imaging the distribution and secondary structure of immobilized enzymes using infrared microspectroscopy. Biomacromolecules 4:70–74
Rathi P, Saxena RK, Gupta R (2001) A novel alkaline lipase from Burkholderia cepacia for detergent formulation. Process Biochem 37:187–192
Rehm BHA (2003) Polyester synthases: natural catalysts for plastics. Biochem J 376:15–33
Rehm BHA (2006) Genetics and biochemistry of polyhydroxyalkanoate granule self-assembly: the key role of polyester synthases. Biotechnol Lett 28:207–213
Sheldon RA (2007) Enzyme immobilization: the quest for optimum performance. Adv Synth Catal 349:1289–1307
Svendsen A, Clausen IG, Patkar SA, Borch K, Thellersen M (1997) Protein engineering of microbial lipases of industrial interest. Methods Enzymol 284:317–340
Tan T, Hu J, Nie K, Deng L, Wang F (2010) Biodiesel production with immobilized lipase: a review. Biotechnol Adv 28:628–634
Uppenberg J, Hansen MT, Patkar S, Jones TA (1994) The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida antarctica. Sequence 2:293–308
Acknowledgments
This work was funded by PolyBatics Ltd.
Conflict of interest
Bernd Rehm is Chief Science Officer as well as shareholder of Polybatics Ltd.
Supporting information
Supplementary Table 1—Bacterial strains, plasmids and oligonucleotides used in this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jahns, A.C., Rehm, B.H.A. Immobilization of active lipase B from Candida antarctica on the surface of polyhydroxyalkanoate inclusions. Biotechnol Lett 37, 831–835 (2015). https://doi.org/10.1007/s10529-014-1735-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1735-7