Abstract
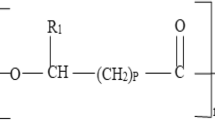
Glyceryl ferulate was synthesized by the condensation of ferulic acid with glycerol using Pectinase PL “Amano” from Aspergillus niger, which contained ferulic acid esterase, to improve the water-solubility of ferulic acid. The optimum reaction medium was glycerol/0.1 M acetate buffer, pH 4.0, (98:2 v/v). The enzyme immobilized onto Chitopearl BCW3003 exhibited the highest activity among the those immobilized onto various kinds of Chitopearl BCW resins. The optimum temperature for the immobilized enzyme was 50°C, and it could be reused at least five times without a significant loss in activity for the synthesis of glyceryl ferulate in batch reaction. Storage of the reaction mixture at 25°C improved the molar fraction of glyceryl ferulate relative to the dissolved ferulic residues.





Similar content being viewed by others
References
Andreasen MF, Kroon PA, Williamson G, Garcia-Conesa M-T (2001) Intestinal release and uptake of phenolic antioxidant diferulic acids. Free Radical Biol Med 31:304–314
Cho JY, Kim HS (2005) Inhibitory effects of long-term administration of ferulic acid on astrocyte activation induced by intracerebroventricular injection of β-amyloid peptide (1–42) in mice. Progr Neuro-Psychopharm Biol Psychol 29:901–907
Fang X, Shima M, Kadota M, Tsuno T, Adachi S (2006) Suppressive effect of alkyl ferulate on the oxidation of linoleic acid. Biosci Biotechnol Biochem 70:457–461
Graf E (1992) Antioxidant potential of ferulic acid. Free Radical Biotechnol Med 13:435–448
Kobayashi T, Adachi S, Matsuno R (2003) Kinetic analysis of the immobilized-lipase-catalyzed synthesis of octanoyl octyl glucoside in acetonitrile. Biochem Eng J 16:323–328
Stamatis H, Sereti V, Kolisis FN (2001) Enzymatic synthesis of hydrophilic and hydrophobic derivatives of natural phenolic acids in organic media. J Mol Catal B Enzym 11:323–328
Tsuchiyama M, Sakamoto T, Fujita T, Murata S, Kawasaki H (2006) Esterification of ferulic acid with polyols using a ferulic acid esterase from Aspergillus niger. Biochim Biophys Acta – Gen Subj 1760:1071–1079
Wende G, Fry SC (1997a) O-feruloylated, O-acetylated oligosaccharides as side-chains of grass xylans. Phytochemistry 44:1011–1018
Wende G, Fry SC (1997b) 2-O-β-d-xylopyranosyl-(5-O-feruloyl)-l-arabinose, a widespread component of grass cell walls. Phytochemistry 44:1019–1030
Yoshida Y, Kimura Y, Adachi S (2006a) Thermal inactivation of immobilized lipase in 1-alcohols. J Biosci Bioeng 102:66–68
Yoshida Y, Kimura Y, Kadota M, Tsuno T, Adachi S (2006b) Continuous synthesis of alkyl ferulate by immobilized Candida antarctica lipase at high temperature. Biotechnol Lett 28:1471–1474
Acknowledgment
Pectinase PL “Amano” was supplied by Amano Enzyme Co., Ltd., Nagoya, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsuo, T., Kobayashi, T., Kimura, Y. et al. Synthesis of glyceryl ferulate by immobilized ferulic acid esterase. Biotechnol Lett 30, 2151–2156 (2008). https://doi.org/10.1007/s10529-008-9814-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-008-9814-2