Abstract
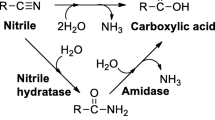
2,6-Pyridinedicarbonitrile (1a) and 2,4-pyridinedicarbonitrile (2a) were hydrated by Rhodococcus erythropolis A4 to 6-cyanopyridine-2-carboxamide (1b; 83% yield) and 2-cyanopyridine-4-carboxamide (2b; 97% yield), respectively, after 10 min. After 118 h, the intermediates 1b or 2b were transformed into 2,6-pyridinedicarboxamide (1c; 35% yield) and 2,6-pyridinedicarboxylic acid (1d; 60% yield) or 2-cyanopyridine-4-carboxylic acid (2c; 64% yield), respectively. The nitrilase from Fusarium solani afforded cyanocarboxylic acids 1e and 2c after 118 h (yields 95 and 62%, respectively). 3,4-Pyridinedicarbonitrile (3a) and 2,3-pyrazinedicarbonitrile (4a) were inferior substrates of nitrile hydratase and nitrilase.



Similar content being viewed by others
References
Čejková A, Masák J, Jirků V, Veselý M, Pátek M, Nešvera J (2005) Potential of Rhodococcus erythropolis as a bioremediation organism. World J Microbiol Biotechnol 21:317–321
Effenberger F, Osswald S (2001) Enantioselective hydrolysis of (RS)-2-fluoroarylacetonitriles using nitrilase from Arabidopsis thaliana. Tetrahedron: Asymmetry 12:279–285
Kaplan O, Nikolaou K, Pišvejcová A, Martínková L (2006) Hydrolysis of nitriles and amides by filamentous fungi. Enzyme Microb Technol 38:260–264
Kaplan O, Vejvoda V, Plíhal O, Pompach P, Kavan D, Bojarová P, Bezouška K, Macková M, Cantarella M, Jirků V, Křen V, Martínková L (2006) Purification and characterization of a nitrilase from Aspergillus niger K10. J Appl Microbiol Biotechnol 73:567–575
Kiziak C, Conradt D, Stolz A, Mattes R, Klein J (2005) Nitrilase from Pseudomonas fluorescences EBC 191: cloning and heterologous expression of the gene and biochemical characterization of the recombinant enzyme. Microbiology 151:3639–3648
Martínková L, Klempier N, Přepechalová I, Přikrylová V, Ovesná M, Griengl H, Křen V (1998) Chemoselective biotransformation of nitriles by Rhodococcus equi A4. Biotechnol Lett 20:909–912
Martínková L, Mylerová V (2003) Synthetic applications of nitrile-converting enzymes. Curr Org Chem 7:1279–1295
Přepechalová I, Martínková L, Stolz A, Ovesná M, Bezouška K, Kopecký J, Křen V (2001) Purification and characterization of the enantioselective nitrile hydratase from Rhodoccoccus equi A4. Appl Microbiol Biotechnol 55:150–156
Stevenson DE, Feng R, Dumas F, Groleau D, Mihoc A, Storer AC (1992) Mechanistic and structural studies on Rhodococcus ATCC 39484 nitrilase. Biotechnol Appl Biochem 15:283–302
Sugai T, Yamazaki T, Yokoyama M, Ohta H (1997) Biocatalysis in organic synthesis: the use of nitrile- and amide-hydrolyzing microorganisms. Biosci Biotech Biochem 61: 1419–1427
Vejvoda V, Kaplan O, Klozová J, Masák J, Čejková A, Jirků V, Stloukal R, Martínková L (2006) Mild hydrolysis of nitriles by Fusarium solani strain O1. Folia Microbiol 51:251–256
Vejvoda V, Kaplan O, Kubáč O, Křen V, Martínková L (2006) Immobilization of fungal nitrilase and bacterial amidase – two enzymes working in accord. Biocatal Biotrans 24:414–418
Acknowledgement
Financial support through projects IAA500200708 (Grant Agency of the Academy of Sciences of the Czech Republic), LC06010 and OC D25.001 (Ministry of Education, Czech Republic) and the institutional research concept AV0Z50200510 (Institute of Microbiology) is gratefully acknowledged. We also wish to thank Mrs. Jana Horová for her excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
6-Cyanopyridine-2-carboxamide (1b) 1H NMR (399.87 MHz, DMSO-d 6 , 30°C): 7.830 (1 H, br s, NH2-u), 8.211 (1H, dd, J = 7.6, 2.2 Hz, H-5), 8.22 (1H, br s, NH2-d), 8.238 (1 H, dd, J = 7.6, 7.0 Hz, H-4), 8.294 (1H, dd, J = 7.0, 2.2 Hz, H-3). 13C NMR (100.55 MHz, DMSO-d 6 , 30°C): 117.09 (6-CN), 125.88 (C-3), 131.31 (C-5), 131.46 (C-6), 139.75 (C-4), 151.91 (C-2), 164.49 (2-CO).
6-Cyanopyridine-2-carboxylic acid (1e) 1H NMR (399.87 MHz, DMSO-d 6 , 30°C): 8.231 (2 H, m, H-4, H-5), 8.296 (1 H, m, H-3). 13C NMR (100.55 MHz, DMSO-d 6 , 30°C): 117.01 (6-CN), 128.21 (C-3), 131.64 (C-5), 132.57 (C-6), 139.63 (C-4), 150.23 (C-2), 164.77 (2-CO).
2-Cyanopyridine-4-carboxamide (2b) 1H NMR (399.87 MHz, DMSO-d 6 , 30°C): 7.948 (1 H, br s, NH), 8.078 (1 H, dd, J = 5.1, 1.7 Hz, H-5), 8.347 (1 H, dd, J = 1.7, 0.9 Hz, H-3), 8.370 (1 H, br s, NH), 8.904 (1 H, dd, J = 5.1, 0.9 Hz, H-6). 13C NMR (100.55 MHz, DMSO-d 6 , 30°C): 117.29 (2-CN), 125.49 (C-5), 126.69 (C-3), 133.26 (C-2), 142.92 (C-4), 152.11 (C-6), 164.55 (4-CO).
2-Cyanopyridine-4-carboxylic acid (2c) 1H NMR (399.87 MHz, DMSO-d 6 , 30°C): 8.079 (1 H, dd, J = 4.9, 1.3 Hz, H-5), 8.296 (1 H, s, H-3), 8.872 (1 H, d, J = 4.9 Hz, H-6). 13C NMR (100.55 MHz, DMSO-d 6 , 30°C): 117.37 (2-CN), 126.85 (C-5), 128.05 (C-3), 133.25 (C-2), 143.11 (C-4), 152.07 (C-6), 164.82 (4-CO).
Rights and permissions
About this article
Cite this article
Vejvoda, V., Šveda, O., Kaplan, O. et al. Biotransformation of heterocyclic dinitriles by Rhodococcus erythropolis and fungal nitrilases. Biotechnol Lett 29, 1119–1124 (2007). https://doi.org/10.1007/s10529-007-9364-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-007-9364-z