Abstract
Spinal muscular atrophy (SMA) is one of the most prevalent autosomal recessive illnesses with type I being the most severe type. Genomic alterations including survival motor neuron (SMN) copy number as well as deletions in SMN and Neuronal Apoptosis Inhibitory Protein (NAIP) are greatly implicated in the emergence of SMA. However, the association of such alterations with the severity of the disease is yet to be investigated. This study was directed to elucidate the molecular assessment of NAIP and SMN genomic alterations as a useful tool in predicting the severity of SMA among patients. This study included 65 SMA pediatric patients (30 type I and 35 type II) and 65 healthy controls. RFLP-PCR was employed to determine the genetic polymorphisms of the SMN1, SMN2, and NAIP genes. In addition, qRT-PCR was used to identify the expression of the SMN1 and SMN2 genes, and serum levels of creatine kinase were measured using a colorimetric method. DNA sequencing was performed on some samples to detect any single nucleotide polymorphisms in SMN1, SMN2, and NAIP genes. All SMA patients had a homozygous deficiency of SMN1 exon 7. The homozygous deficiency of SMN1 exons 7 and 8, with the deletion of NAIP exon 5 was found among the majority of Type I patients. In contrast, patients with the less severe condition (type II) had SMN1 exons 7 and 8 deleted but did not have any deletions in NAIP, additionally; 65.7% of patients had multiple copies of SMN2. Analysis of NAIP deletion alongside assessing SMN2 copy number might enhance the effectiveness of the diagnosis that can predict severity among Spinal Muscular Atrophy patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal muscular atrophies (SMA) comprise a group of neuromuscular disorders characterized by degeneration of alpha motor neurons in the spinal cord with progressive muscle atrophy, weakness, and paralysis (Srivastava and Srivastava. 2019). It encompasses a broad spectrum of phenotypes that are divided into clinical groups based on the age of onset and the maximum motor function attained: very weak infants unable to sit unsupported (type I), non-ambulant patients able to sit independently (type II), up to ambulant patients with childhood (type III), and adult-onset SMA (type IV) (Mercuri et al. 2018).
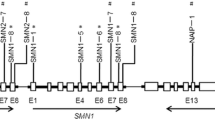
The survival motor neuron (SMN) gene, is the most frequently affected gene in SMA (Mercuri et al. 2018). Two extremely homologous copies of the SMN genes, the telomeric (SMN1) and the centromeric (SMN2) are known. Although, mutations in the SMN1 but not SMN2 are the cause of SMA, the expression of SMN2 is connected to the clinical phenotype of SMA, where patients with less severe types of SMA have more copies of the SMN2 gene than do patients with the more severe types of the disease (Niba et al. 2021). A single nucleotide in each exon 7 and exon 8 of the SMN1 and its highly homologous copy, i.e., SMN2, changes the restriction enzyme digestion patterns of the two genes; making it possible to differentiate the SMN1 from SMN2 using a restriction fragment length polymorphism PCR (RFLP-PCR) (Alimanović and Šutković. 2020).
On chromosome 5q13, there is a 500 kb inverted duplication that includes the Neuronal Apoptosis Inhibitory Protein (NAIP) gene which is located near the SMN1 gene. Recently, NAIP has been suggested to have a key role in the development of SMA (Smeriglio et al. 2020). Moreover, a genotype with less copies of combination of SMN1-SMN2-NAIP is linked to earlier onset age and lower survival probability (Qu et al. 2015).
Creatine kinase (CK), a substance produced during muscle degeneration and leaks out into the blood stream, hence it can be used for measuring muscle degeneration. Although elevated blood CK levels doesn't necessarily have clinical implications, increased CK levels reflect the presence of muscle injury or degeneration. CK levels are typically normal in people with SMA type I but may occasionally be slightly higher in people with types II and III of the disease (De Wel et al. 2021).
Few previous studies were carried out to investigate genomic alterations of SMN1 and NAIP genes in the Egyptian population (Essawi et al. 2007; Shawky et al. 2001), however these studies lacked the combined analysis of the SMN1, SMN2, and NAIP genes in the same study, and no study highlighted the association of such alterations with the severity of the disease. Therefore, the present study aimed to study SMA disease (type I and II) in pediatric patients in Egypt at the molecular level, and elucidate the possibility of using the molecular assessment of NAIP and SMN genes as a useful tool in predicting the severity of SMA among patients.
Materials and Methods
Patients
The Sampling procedures and study design of the present work were reviewed and approved by Ethical Committee in Medical Research Institute, Alexandria University, and a signed consent forms were collected from all the participants prior to enrollment in the study. This study enrolled sixty-five healthy control and sixty-five pediatric patients with SMA (30 patients with type I and 35 patients with Type II), the patients chosen from those who were admitted to the Pediatric Neurology Department in Alexandria Pediatric Hospital. The inclusion criteria of the patients were based on clinical examination and investigations including EMG, nerve conduction velocity.
From all participants, two blood samples were withdrawn, one into a plain tube while the other one in EDTA-coated tube, for collecting serum and whole blood, respectively. All samples were stored at −80 °C until the time of investigations.
Methods
Colorimetric Determination of Serum Creatine Kinase (CK)
Serum levels of CK were determined using creatine kinase (CK) kit (BIOLABO, France). The assay method using creatine phosphate and Adenosine diphosphate (ADP) (Wu. 2006).
Determination of the Genetic Polymorphisms of SMN1, SMN2, and NAIP Genes by RFLP-PCR
Genomic DNA Extraction From Whole Blood
Genomic DNA was extracted from 200 μl of whole blood using G-spin™ Total DNA Extraction Mini Kit (iNtRON Biotechnology, Korea), following manufacture instructions. The concentration and the purity of the extracted DNA were determined by measuring absorbance at 260 and 280 nm using spectrophotometer (Wilfinger et al. 1997). The extracted DNA had concentrations ranged between 100–200 ng/µl, with 1.7 – 1.9 purity range. Then this DNA was stored at −20 °C till it was used in further genetic analysis, such as RFLP-PCR technique and DNA sequencing.
Detection of the Deletion of Exon 5 of the NAIP Gene
Deletion of exon 5 of the NAIP gene (unique to the functional copy of NAIP gene) was detected using a Multiplex Polymerase Chain Reaction (multiplex-PCR) (Stewart et al. 1998). Exon 13 of the NAIP gene amplification was used as an internal control to distinguish the actual deletion of exon 5 from failure of amplification. All primer sequences used for RFLP-PCR are represented in (Table 1).
DNA was amplified by multiplex PCR using BIO-RAD T100 Thermocycler. The reaction was carried-out in a final volume of 40 µl consisting of 20 µl of 2 × Power PCR Master Mix (Thermo Fischer Scientific, USA), 100 ng DNA template, 0.5 µmol from each primer for exon 5 and 13 (NAIP5F/R and NAIP13F/R). The thermal profile was as follows: 5 min initial denaturation at 95 °C, followed by 35 cycles of denaturation for 30 s at 95 °C, annealing at 60 °C for 45 s, and extension at 72 °C for 45 s, then final extension at 72 °C for 10 min. Then, the PCR products were analyzed on a 2% agarose gel containing ethidium bromide, (Supplementary file Fig. 1).
Detection of the Absence of Exons7 and 8 of the SMN1 Gene
DNA was amplified by multiplex PCR using BIO-RAD T100 Thermocycler. The reaction was carried-out in a final volume of 40 µl consisting of 20 µl of 2 × Power PCR Master Mix (Thermo Fischer Scientific, USA), 50 ng DNA template, 2 μl from each primer (SMN7F) or (SMN8F), and 2 μl from each primer (SMN7R) or (SMN8R). The thermal conditions were as follows: 5 min initial denaturation at 95 °C, followed by 38 cycles of denaturation for 30 s at 95 °C, annealing for 45 s at 56 °C (exon 7) or 59 (exon 8), and extension at 72 °C 45 s, then final extension at 72 °C for 10 min. (Shin et al. 2000; Scheffer et al. 2001).
For detection of exon 7 deletion, 20 μl of PCR product were incubated with 5U of the restriction enzyme (Dra1), for 4 h at 37 ºC then terminated by keeping at -20 ºC for 10 min. 20 µl from each tube were electrophoresed on 2.5% agarose gel electrophoresis. The restriction patterns of SMN1 exon 7 gene were visualized by gel documentation system after staining with 10 mg/ml of ethidium bromide, (Supplementary file Fig. 2). At position 875 of the DNA, we induced a G to A alteration (G > A) by employing a mismatch primer at the 3' end of the exon 7. A Dra1 site is created by this alteration (TTTAAA). The enzyme selectively cuts the SMN2 amplified PCR product because the third T nucleotide of the sequence is only found in the SMN2 gene and is a C in the SMN1 gene, the subsequent digestion products were anticipated (Alimanović and Šutković. 2020). Individuals with a normal SMN1 gene or those who carry a heterozygous SMN1 deletion exhibit both, an undigested product of approximately 200 bp and a digested product of 176 bp for the SMN1 and SMN2 genes, respectively. Conversely, individuals who lack the SMN2 gene (which accounts for 5% of the normal population) should only produce the larger 200 bp PCR product indicative of the SMN1 gene. In affected patients, only the SMN2 gene is expected, which is represented by the 176 bp PCR product, whereas the 200 bp product indicating the SMN1 gene is absent (Alimanović and Šutković. 2020).
For RFLP analysis of exon 8 deletion, 20 μl PCR product were incubated with 5U of (Dde1) restriction enzyme for 4 h at 37 ºC then terminated by keeping it at -20 ºC for 10 min. 20 µl from each tube was electrophoresed on a 2.5% agarose gel. The restriction patterns of SMN1 exon 8 gene were visualized by gel documentation system after staining with 10 mg/ml of ethidium bromide, (Supplementary file Fig. 3). The nucleotide change from G to A is what allows for distinguishing between the SMN1 and SMN2 genes. (Blasco‐Pérez et al. 2021). Presence of an A nucleotide in the SMN2 gene at DNA position 1155 creates a Dde1 site, which cleaves the amplified DNA into two fragments of approximately 122 bp and 78 bp. The amplified product of exon 8 from the SMN1 gene, which is 200 bp in length, does not contain any Dde1 sites. Following amplification and digestion of exon 8, the following restriction pattern is expected. For individuals with a normal SMN1 gene or those who carry a heterozygous SMN1 deletion, the expected outcome would be a 200 bp PCR product representing the SMN1 gene along with digestion products of 122 bp and 78 bp representing the SMN2 gene. In those who lack the SMN2 gene, only the undigested product (200 bp) indicating the SMN1 gene is expected. In affected individuals, only the SMN2 gene is expected, resulting in digestion products of 122 bp and 78 bp. The 200 bp PCR product representing the SMN1 gene would not be present in this case (Alimanović and Šutković. 2020).
Determination of the Expression of SMN1 and SMN2 Genes by Reverse-Transcriptase PCR (qRT-PCR)
The expression of SMN1 and SMN2 genes was quantified using qRT-PCR which employed an intercalating dye (SYBR Green), which binds indiscriminately to all double stranded DNA products. A melt-curve analysis were performed to assure the absence of any nonspecific products or primer dimmers.
RNA Extraction
In brief, total RNA was extracted from 200 μl of whole blood using Easy-REDTM Total RNA Extraction Kit (# 17,063, iNtRON Biotechnology Inc., South Korea) according to the manufacturer’s instructions. The concentration and the purity of RNA were determined by measuring absorbance at 260 and 280 nm using spectrophotometer (Wilfinger et al. 1997).
cDNA Synthesis & Amplification
Full-length cDNA from a total RNA samples were synthesized using the HiSenScript™ RH (-) cDNA Synthesis Kit (# 25,087, iNtRON Biotechnology Inc., South Korea) according to the manufacturer’s protocol. The PCR reaction was performed in final volume of 20 μl consisting of 10 μl qPCR 2X PreMIX (SYBR Green with low ROX), 1 µl cDNA, 1 μl from primer (SMN1qF) or (SMN2qF) with (GAPDHF) and 1 μl from primer (SMN1qR) or (SMN2qR)) with (GAPDHR) and 7 µl RNase-free water. The thermal profile was as follows: 12 min initial denaturation at 95 °C, followed by 40 cycles of denaturation for 10 s at 95 °C, annealing for 15 s at 57 °C (exon 7) or 59 (exon 8), and extension at 72 °C for 30 s, then final extension at 72 °C for 10 min. Table. 1 indicates the primer sequences used for RT-PCR.
The threshold cycle (Ct) values for each sample were determined as the number of cycles at which fluorescent emission first exceeded the baseline value. The difference of the Ct value (ΔCt) between target gene and housekeeping gene (GAPDH) for each sample was calculated and the calibrated ΔCt value (ΔΔCt) for each sample was calculated (ΔΔCt = ΔCt control sample – ΔCt patient sample). The relative gene copy number was calculated by the expression 2−ΔΔct. Using this method, a ΔΔCt ratio (2−ΔΔCt) \({2}^{-\Delta \Delta Cq}\) of SMN1 was expected to be about 1 in normal control, about 0.5 in carriers and 0 in patients with SMA (Lee et al. 2004).
DNA Sequencing
DNA sequencing was performed for SMN1, SMN2, and NAIP genes amplicon for selected 5 controls and 10 patients. DNA was amplified by PCR using Applied Biosystems Thermocycler. The reaction was carried-out in a final volume of 25 µl consisting of 5 µl reaction Buffer (5X), 0.5 µl dNTP (10 mM), 1.25 µl forward/reverse primers (10 µM), and 0.25 µl high-fidelity DNA polymerase (Thermo Fischer Scientific, USA), 100 ng DNA template, the thermal profile for each gene was performed as mentioned earlier. Sequencing of the PCR product using Sanger method was carried-out on 3730xl Genetic Analyzer, ABI Systems, and the reaction was performed using BigDye® v3.1 (Life Technologies, Applied Biosystems) as per the manufacturer’s protocol. Signal detection was done using 3730 Data collection software and sequencing analysis software v5.0.
Finally, the sequencing results of controls and patients’ samples for SMN1, SMN2, and NAIP genes fragments were analyzed by MEGA 5.05 (1993–2011) and Blast 2.0 software to detect the possible SNPs between sequenced samples. DNA sequences alignment was compared among the sequenced fifteen selected samples (controls and patients) in addition to the available sequences in the GenBank.
Statistical Analysis of the Data
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp) Qualitative data were described using number and percent. The Kolmogorov–Smirnov test was used to verify the normality of distribution Quantitative data were described using range (minimum and maximum), mean, standard deviation, median, and interquartile range (IQR). Significance of the obtained results was judged at the 5% level. Chi-square test was used to compare categorical variables between different groups. Monte Carlo correction was done for correction for chi-square when more than 20% of the cells have expected count less than 5. Kruskal Wallis test was used for abnormally distributed quantitative variables, to compare between more than two groups, and Post Hoc (Dunn's multiple comparisons test) was done for pairwise comparisons. Mann Whitney test was performed for abnormally distributed quantitative variables, to compare between two studied groups.
Results
Patients’ Characteristics
Based on the clinical, radiological, and biochemical tests, SMA patients enrolled in the present study were classified into two groups type I and Type II. The first group (SMA type I) included 30 patients (46%), with age range (0.17 – 1.17) and the mean age of onset was 0.63 ± 0.27 years. Whereas the second group (SMA type II) included 35 patients (54%) with age range (range 0.67 – 13.0) and the mean age of onset was 5.10 ± 3.56 years. The healthy control group includes 65 subjects with age range 0.17 – 13.0 and mean age of 3.13 ± 3.50 (Table 2).
Serum Levels of CPK
The results of the present study revealed the lack of any significant difference between serum levels of CPK in SMA patients (type I and Type II) and the healthy controls (Fig. 1).
Detection of Gene Deletion in SMN1, SMN2, and NAIP Genes Using RFLP-PCR
No homozygous deletions were seen in any of the enrolled healthy control subjects. The homozygous deletion of SMN1 exon 7 was found in 65 out of 65 SMA patients (100%). SMN1 exon-7 only deletion was found in 2 of 30 type I patients (6.7%) and in 4 of 35 type II patients (11.4%). Homozygous deletions of both exons 7 and 8 in SMN1 were seen in 90% of type I and 88.6% of type II SMA Patients (Table 3).
With regard to the NAIP gene, deletion of exon 5 was detected in 25 out of 65 SMA patients (38.46%). Where, in type I SMA, deletion was found in 17 of 30 patients (56.7%) and in 8 of 35 type II patients (22.9%) (Table 3). On the other side, homozygous deletions of both exons 7, 8 in SMN1 and NAIP exon 5 were seen in 53.3% of type I and 22.9% of type II SMA Patients (Table 3).
SMN1 Copy Number and Gene Expression
In healthy control subjects, the relative gene copy number of SMNI gene range was 1.0 – 3.0 with a mean of 1.98. On the other hand, the relative gene copy number was (0.0) in SMA patients and SMN1 gene was not amplified reflecting homozygous absence of SMN1 gene. Moreover, there was a significant difference in relative gene copy number between type I and II patients and control group (P < 0.001) (Fig. 2 and Table 4).
SMN2 Copy Number and Gene Expression
The relative gene copy number of SMN2 gene ranged between 0.0 – 2.0 with a mean of 1.83 in the healthy control subjects. Whereas in SMA type I, the relative gene copy number ranged between 1.0 and 3.0 with a mean of 1.60, and between 2.0 and 4 with a mean 3.0 in SMA type II, Table 4. Moreover, there was a significant difference in the relative gene copy number between patients and control group (P < 0.001), and between SMA type I patients and SMA type II group (P < 0.001) (Table 4).
Furthermore, the relative expression of SMN2 gene was significantly upregulated in SMA type I and II as compared to healthy control, and in SMA type II as compared to type I patients, (p ≤ 0.05) (Fig. 2).
Distribution of SMN1 and SMN2 Copy Number among Studied Groups
The distributions of SMN1 copy number in the healthy control were as follows: 1 (3.1%), 2 (94%), and 3 (3.1%) of the controls, whereas it was 0 (100%) in patients’ group. Regarding SMN2 copy number, in the healthy controls; the distributions of SMN2 copy number were as follows: 0 (3.1%), 1 (10.8%), 2 (83.1%) and 4 (3.1%). On the other hand, in SMA type I, the distributions of SMN2 copy number were as follows: 1 (46.7%), 2 (46.7%), and 3 (6.7%), whereas distributions were 2 (20%), 3 (60%), and 4 (20%) in SMA type II (Table 4).
Relation between SMN1, SMN2 Copy Number and Gene Deletion Test
A positive relation was found between the SMN2 copy number and all studied deletion tests in SMA type I&II patients (Tables. 5 and 6).
Confirmation of SNPs by DNA Sequencing
The different detected SNPs in SMN1, SMN2, and NAIP genes are summarized in (Table 7). A total of 4 SNPs in SMN1, namely C -859G was found in SMA patients as (Type I equals two copies of SMN2, while Type II equals three copies of SMN2). G-711A was found to be most significant with identification in SMA type II = 3 copies of SMN2, T-1040A was found to be most significant with identification in SMA type I = 2 copies of SMN2, and T-1058A was found in SMA patients as (Type 1 equals two copies of SMN2, while Type II equals three copies of SMN2). Four other variants that are also usually in SMN2, G-1102C, G-1119C, G-1093 T, and G-1094 T was found to be most significant with identification in SMA type I = 2 copies. A total of 3 SNPs in NAIP, namely G-711A was found to be most significant with identification in SMA type II = 3 copies of SMN2, T-1040A was found to be most significant with identification in SMA type I = 1 copy of SMN2 with NAIP 5 deletion and T-1058A was found in SMA patients as (Type 1 equals two copies of SMN2, while Type II equals three copies of SMN2).
Distribution of the Detected SNPs among SMA Phenotypes
The distribution of different SNPs among different phenotypes in control, SMA type I, and SMA type II related to SMN1, SMN2, and NAIP genes is represented in (Table 8).
Combinations of Genotypes and Associated Phenotypes
The different combinations of SMA genotypes and the associated phenotypes in the populations under study are shown in (Table 9) and (Fig. 3a, b).
Discussion
Spinal muscular atrophy (SMA) is identified as a diverse collection of uncommon, genetically inherited neuromuscular illnesses, it is a crippling condition that frequently renders a person incapable of walking, sitting, eating, speaking, or breathing, and in the most extreme cases, results in paralysis at birth or shortly thereafter and early death. The most defining features of the disease are motor neuron (MN) abnormalities of the ventral horn of the spinal cord, leading to gradual skeletal muscle weakness and consequent atrophy (López-Cortés et al. 2022).
Clinical subtypes of SMS are classified according to the greatest motor milestone attained and the age at which symptoms first appeared. Patients with SMA type I are never able to sit down, whereas those with SMA type I can learn to sit but will never be able to walk unassisted. SMA type III patients learn to stand and walk on their own, although they may lose this ability with time (Coratti et al. 2020). The clinical diagnosis of SMA is often confirmed through the laboratory investigations as well as the molecular diagnosis. The laboratory investigations include the electromyographical studies and the determination of serum CK levels, which are routine laboratory studies used to evaluate patients with neuromuscular disorders (Martinez-Thompson.2021).
Regarding the electromyographical (EMG) studies, our results revealed that the sixty-five pediatric patients had a neuropathic denervation potential at the level of AHCs. This potential pattern indicates decreased number of motor units and denervation, which is greatly associated with spinal muscular atrophy (Sleutjes et al. 2020). EMG is regarded as an invasive test even though it is effective in the diagnosis of spinal muscular atrophy and frequently utilized as supporting evidence in SMA diagnosis. Its performance in the newborns and young infants is difficult to perform and interpret, making it only useful in the hands of experts (Kaler et al. 2020).
All SMA patients enrolled in this study showed normal levels of serum CK. These results agree with a previous study reported a normal or mild elevation of serum CK levels in spinal muscular atrophy (Pino et al. 2021). Creatine kinase is an integral part of the muscle energy metabolism, which is greatly affected in neuromuscular diseases (Renard. 2015) including spinal and bulbar muscular atrophy (Lombardi et al. 2019). Serum creatine kinase activity (CK) might be a promising marker for disease severity in SMA (Freigang et al. 2021). Additionally, determination of serum CK levels is an important blood test to differentiate patients with suspected myopathy, diseases of the muscles themselves, from those with other neuromuscular disorders including spinal muscular atrophy (Martinez-Thompson.2021).
Molecular diagnosis of SMA has emerged as a useful tool in diagnosis of the disease, overcoming all the difficulties encountered by EMG test in younger patients, and become superior over determination of blood CK levels (Kaler et al. 2020; Renard. 2015). Moreover, molecular diagnosis of SMA by detecting absence of SMN1 exons 7 & 8 has been extensively investigated among different ethnic groups; with high frequencies (87–100%) for homozygous absence of either exons 7 & 8 or exon 7 only of SMN1 gene have been reported.
The present study showed that, regardless of disease severity, 65 of the total 65 patients (100%) have homozygous absence of SMN1 exons 7. Moreover, fifty nine patients (90.7%) were found to have homozygous absence of SMN1 exons 7 & 8, six patients (9.3%) showed homozygous absence of exon 7 only. The frequencies of homozygous absence of exons 7 and 8 were (27/30; 90%) and (31/35; 88.6%), for types I and II, respectively. While the frequencies of homozygous absence of exon 7 only were 6.7% (2/30) and 11.4% (4/35), respectively. This observation agrees with results documented by previous reports (Niba et al. 2021; Freigang et al. 2021; Sharifi et al. 2019).
Deletion of NAIP gene exon 5 was found in 38.46% (25/65) of the SMA patients included in this study. The incidence of deletion was more frequent in type I patients (56.7%; 17/30) as compared to types II (22.9%; 8/35). This result was in agreement with several previous investigations reporting a higher incidence of this genotype among type I SMA patients as compared to patients with type II (Niba et al. 2021; Freigang et al. 2021; Sharifi et al. 2019). Gene deletion studies indicated the presence of homozygous deletions for both exons 7, 8 in SMN1 and exon 5 in 53.3% of type I SMA and 22.9% of type II patients. Furthermore; our results showed that all patients reported with NAIP deletion lacked the SMN1 exon 7 or exon 7 and 8. This is in consistence with several studies which have reported that loss of both copies of NAIP is not sufficient to cause the disease, as 1–2% of unaffected individuals and 2% of carrier parents, showed deleted NAIP gene in both chromosomes have found that in their SMA patients, deletion of NAIP gene was always associated with homozygous deletions of SMN1 gene. However, incidences where types I or II SMA patients with deleted NAIP exon 5 but retaining SMN1 gene have been reported (Niba et al. 2021; Freigang et al. 2021; Sharifi et al. 2019). Suggestions for the occurrence of such a genotype included possible presence of other types of mutations in either SMN1 or in other unknown genes and /or the possibility that NAIP deletion is not always associated with SMN deletions as had been previously suggested by Mitchell and his team (Hassan et al. 2020).
It is noteworthy that investigating the presence of point mutations (subtle mutations) in SMN1 gene in the enrolled patients would be of great importance in improving molecular diagnosis of the disease. The results of our study revealed the presence of five different mutation patterns among the studied Egyptian patients; including Mutation pattern A, B, C, D, and E.
In mutation pattern (A), the alleles carrying homozygous absence of SMN1 exons 7 & 8 with deletion of NAIP exon 5 are considered as severe alleles (Mitchell et al. 2020). These alleles indicate large-scale deletions that remove the entire coding region of SMN1 gene as well as the intact NAIP gene. In this case deletion of other modifier genes, like p44 and H4F5, is expected. In the present study, homozygous absence of SMN1 exons 7 & 8 in association with deletion of NAIP exon 5 were found in most type I patients (16/30; 53.3%) and in smaller percent in type II (8/35; 22.9%) SMA patients the distributions of SMN1 copy number were 0. The distribution of SMN2 copy numbers were as follows: 1(50%), 2(50%) with type I and. The distribution of SMN2 copy numbers were as follows: 2(12.5%), 3(62.5%), and 4 (25%) with type II.
These results are in agreement with a previous report indicating a higher incidence of this genotype among type I SMA patients as compared to patients with types II. The higher percent of this pattern in some type III patients compared to type II was unexpected because large-scale deletions are associated with severe alleles and consequently with a more severe disease phenotype (Zhang et al. 2020). However, this result is consistent with the presence of other possible unknown factors that might influence the genotype–phenotype correlation in SMA patients (Blasco-Pérez et al. 2022; Kekou et al. 2020). On the other hand, our results disagreed with the previous reported studies in which the minority of type I patients carried this genotype (Vijzelaar et al. 2019).
The second mutation pattern is B involves alleles with homozygous absence of both SMN1 exons 7 & 8 with retention of NAIP gene. In the present investigation this genotype was found in (11/30; 36.7%) and (23/35; 65.7%) of types I and II, respectively. The distributions of SMN1 copy number were 0. The distributions of SMN2 copy number were as follows: 1(54.5%), 2(36.4%), and 3(9.1%) with type I and the distributions of SMN2 copy number were as follows: 2(26%), 3(52%), and 4 (22%) with type II. All these results indicate that deletions including SMN1 but not NAIP genes are associated with milder phenotype of the disease. In contrast, a previous study reported a higher frequency of this mutation pattern in type I patients than in types II/III of South African Black patients (Vijzelaar et al. 2019).
Patients with mutation pattern C are characterized with homozygous absence of SMN1 exon 7 only has been more frequently associated with minorities of types II patient as compared to type I. In the present investigation, this genotype was found in (2/30; 6.7%) and (4/35; 11.4%) of types I and II, respectively. The distribution of SMN1 copy number were 0. The distributions of SMN2 copy number were as follows: 2(50%) and 3(50%) with type I and the distributions of SMN2 copy number were as follows 3(100) with type II. Vijzelaar and his group have reported that the homozygous absence of SMN1 exon 7 only in a remarkably higher frequency in types II (Wirth et al. 2020). Surprisingly Wirth and his colleagues have reported such a mutation pattern in type I patients only (Butchbach. 2021). Homozygous absence of SMN1 exon 7 and retention of exon 8 in type I patients has been postulated to be the result of unequal crossing over, while that found in types II and III has been considered the result of SMN1 gene conversion into SMN2 (Kekou et al. 2020). Gene conversion events have been reported to account for the minority of SMA patients with a frequency of 3–28% (Yuan and Jiang. 2015).
The mutation pattern D involves homozygous absence of SMN1 exon 7 with deleted NAIP exon 5 and retention of SMN1 exon 8. In the present investigation, only one type I patient (1/30; 3.3%) was found carrying such a pattern. This pattern was absent in type II patients. The distributions of SMN1 copy number were 0. The distributions of SMN2 copy number were as follows: 2(100%) with type I. The occurrence of this genotype is somehow intriguing with respect to the known gene order [SMN2-SMN1-NAIP]. A two steps mechanism has been proposed (Yuan and Jiang. 2015) in which a hybrid SMN gene is found followed by an NAIP deletion outside the SMN region. On the other hand, considering another possibility of gene organization [SMN2-NAIP- SMN1], the NAIP gene could be lost together with the intervening region between SMN2 intron 7 and SMNl exon 8 during the unequal crossing over or the intrachromosomal deletion process (Niba et al. 2021).
SMA patients with mutation pattern E don’t show either homozygous absence of SMN1 or deletion of NAIP genes belong to mutation pattern E. In the present investigation, this pattern was absent in type I and type II patients. These patients are considered as having a subtle mutation in one or extremely rarely in both SMN1 alleles, or as having SMN1 -unlinked SMA, as suggested in a study byYuan and Jiang (Yuan and Jiang. 2015; Butchbach.2021). For genotype–phenotype correlation studies among SMA patients, it is necessary to first identify whether they carry compound heterozygous mutations or if they are SMN1 -unlinked SMA. Subsequently, subtle mutation(s) should be identified in cases of heterozygousity (Allison et al. 2022) have reported that some of the subtle mutations result in severe phenotypes, while others might be considered as mild mutations according to their effect on the expressed SMN protein.
In this study, we have sequenced the SMN1, SMN2, and NAIP genes on 10 of SMA patients (I&II) and 5 of controls in order to find modifiers of SMA. We classified each patient as (Type I = 1, 2 copies of SMN2, Type II = 3 copies of SMN2 and control = 2 copies of SMN2). The sequencing data was analyzed for variants as well as SMN2 copy number which was then verified using qRT-PCR. From this analysis, we found 10 variants. The SNPs with the highest significance are shown in Table (7). A total of 4 SNPs in SMN1, namely C -859G p (Ala2Gly) was found in SMA patients as (Type I = 2 copies of SMN2 and Type II = 3 copies of SMN2). Causes an amino acid change from Ala to Gly at position 2, this variant has previously been described as disease causing for Spinal muscular atrophy (38), G-711A was found to be most significant with identification in SMA type II = 3 copies of SMN2, T-1040A was found to be most significant with identification in SMA type I = 2 copies of SMN2, and T-1058A was found in SMA patients as (Type I = 2 copies of SMN2 and Type II = 3 copies of SMN2).
Four other variants that are also usually in SMN2, G-1102C, G-1119C, G-1093 T and G-1094 T was found to be most significant with identification in SMA type I = 2 copies. A total of 3 SNPs in NAIP, namely G-711A was found to be most significant with identification in SMA type II = 3 copies of SMN2, T-1040A was found to be most significant with identification in SMA type I = 1 copy of SMN2 with NAIP 5 deletion and T-1058A was found in SMA patients as (Type I = 2 copies of SMN2 and Type II = 3 copies of SMN2). Our analysis of 65 SMA patients corroborates the existence of a strong, inverse correlation between SMN2 copy number and disease severity. Thus, one and four SMN2 copies are the genotypes most closely linked to a particular SMA phenotype: 100% of these individuals suffer from the most life-threatening type of the disease or the milder type III, respectively. In particular, the presence of a single SMN2 copy implies that minimal SMN protein production is tightly linked to particularly severe phenotypes, sometimes referred to as type 0 or type I SMA (Tan et al. 2020; Axente et al. 2022).
In conclusion, our team analyzed and compared gene copy numbers and genetic polymorphisms of SMN1, SMN2 and NAIP genes in a sample of Egyptian SMA Patients and healthy individuals. Based on our results, we can conclude that a close relationship might exist between SMN2 copy number and SMA disease severity, suggesting that the determination of SMN2 copy number may be a good predictor of SMA disease type. Furthermore, NAIP gene deletion was found to be associated with SMA severity. Therefore, combining the analysis of deletion of NAIP with the assessment of SMN2 copy number increases the value of this tool in predicting the severity of SMA. Alleles carrying homozygous absence of SMN1 exons 7 & 8 with deletion of NAIP exon 5 are considered as severe alleles. Finally, the gene structures of SMN and NAIP were also different between the SMA patients and healthy controls, exist and can affect the SMA phenotype. To improve our understanding of genotype–phenotype correlations in SMA patients, it is essential to identify subtle alterations in compound heterozygous patients and quantify the number of SMN2 copies. This approach can provide valuable insights into the relationship between genotype and phenotype in SMA, and help develop more effective diagnostic and therapeutic strategies.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
06 April 2024
The original online version of this article was revised: The Figures 1, 2 and 3 has been moved under the results section.
References
Alimanović A, Šutković J (2020) Polymerase chain reaction detection methods of Survival Motor Neuron genes: a review. Bioeng 1(1):37–43
Allison RL, Khayrullina WE, G, Burnett BG, Ebert AD, (2022) Viral mediated knockdown of GATA6 in SMA iPSC-derived astrocytes prevents motor neuron loss and microglial activation. Glia 70(5):989–1004
Axente M, Mirea A, Sporea C, Pădure L, Drăgoi CM (2022) Clinical and electrophysiological changes in pediatric spinal muscular atrophy after 2 years of nusinersen treatment. Pharmaceutics 14(10):2074
Blasco-Pérez L, Paramonov I, Leno J, Bernal S, Alias L, Fuentes-Prior P, Tizzano EF (2021) Beyond copy number: A new, rapid, and versatile method for sequencing the entire SMN2 gene in SMA patients. Hum Mutat 42(6):787–795
Blasco-Pérez L, Costa-Roger M, Leno-Colorado J, Bernal S, Alias L, Codina-Solà M, Martínez-Cruz D, Tizzano EF (2022) Deep molecular characterization of milder spinal muscular atrophy patients carrying the c. 859G> C Variant in SMN2. Int J Mol Sci 23(15):8289
Butchbach MER (2021) Genomic variability in the survival motor neuron genes (SMN1 and SMN2): Implications for spinal muscular atrophy phenotype and therapeutics development. Int J Mol Sci 22(15):7896
Coratti G, Messina S, Lucibello S, Pera MC, Montes J, Pasternak A, Mercuri E (2020) Clinical Variability in Spinal Muscular Atrophy Type III. Ann Neurol 88(6):1109–1117
De Wel B, Goosens V, Sobota A, Van Camp E, Geukens E, Van Kerschaver G, Claeys KG (2021) Nusinersen treatment significantly improves hand grip strength, hand motor function and MRC sum scores in adult patients with spinal muscular atrophy types 3 and 4. J Neurol 268(3):923–935
Essawi ML, Effat LK, Shanab GM, Al-Ettribi GM, El-Haronui AA, Karim AM (2007) Molecular analysis of SMN1 and NAIP genes in Egyptian patients with spinal muscular atrophy. Bratisl Lek Listy 108(3):133–137
Freigang M, Wurster CD, Hagenacker T, Stolte B, Weiler M, Kamm C, Günther R (2021) Serum creatine kinase and creatinine in adult spinal muscular atrophy under nusinersen treatment. Ann Clin Transl Neurol 8(5):1049–1063
Hassan HA, Zaki MS, Issa MY, El-Bagoury NM, Essawi ML (2020) Genetic pattern of SMN1, SMN2, and NAIP genes in prognosis of SMA patients. Egypt J Med Hum Genet 21(1):1–7
Kaler A, Hussain A, Patel S, Majhi S (2020) Neuromuscular junction disorders and floppy infant syndrome: A comprehensive review. Cureus 12(2):e6922
Kekou K, Svingou M, Sofocleous C, Mourtzi N, Nitsa E, Konstantinidis G, Traeger-Synodinos J (2020) Evaluation of genotypes and epidemiology of spinal muscular atrophy in greece: a nationwide study spanning 24 Years. J neuromuscul dis 7(3):247–256
Lee TM, Kim SW, Lee KS, Jin HS, Koo SK, Jo I, Jung SC (2004) Quantitative analysis of SMN1 gene and estimation of SMN1 deletion carrier frequency in Korean population based on real-time PCR. J Korean Med Sci 19(6):870–873
Lombardi V, Querin G, Ziff OJ, Zampedri L, Martinelli I, Heller C, Fratta P (2019) Muscle and not neuronal biomarkers correlate with severity in spinal and bulbar muscular atrophy. Neurol 92(11):e1205–e1211
López-Cortés A, Echeverría-Garcés G, Ramos-Medina MJ (2022) Molecular pathogenesis and new therapeutic dimensions for spinal muscular atrophy. Biology (Basel) 11(6):894
Martinez-Thompson JM (2021) Electrodiagnostic Assessment of Myopathy Neurol Clinic 39(4):1035–1049
Mercuri E, Finkel RS, Muntoni F, Wirth B, Montes J, Main M, Quijano-Roy S (2018) Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord 28(2):103–115
Mitchell JM, Nemesh J, Ghosh S, Handsaker RE, Mello CJ, Meyer D, Hawes D (2020) Mapping genetic effects on cellular phenotypes with “cell villages.” Biorxiv. https://doi.org/10.1101/2020.06.29.174383
Niba ETE, Nishio H, Wijaya YOS, San Lai P, Tozawa T, Chiyonobu T, Takeshima Y (2021) Clinical phenotypes of spinal muscular atrophy patients with hybrid SMN gene. Brain Dev 43(2):294–302
Pino MG, Rich KA, Kolb SJ (2021) Update on Biomarkers in Spinal Muscular Atrophy. Biomark Insights 16:117727192–211035643
Qu YJ, Ge XS, Bai JL, Wang LW, Cao YY, Lu YY, Song F (2015) Association of copy numbers of survival motor neuron gene 2 and neuronal apoptosis inhibitory protein gene with the natural history in a Chinese spinal muscular atrophy cohort. J Child Neurol 30(4):429–436
Renard D (2015) Serum CK as a guide to the diagnosis of muscle disease. Pract Neurol 15(2):121
Scheffer H, Cobben JM, Matthijs G, Wirth B (2001) Best practice guidelines for molecular analysis in spinal muscular atrophy. Eur J Med Genet 9(7):484–491
Sharifi Z, Forouzesh F, Taheri M, Zeinali S (2019) Constraints of carrier screening in spinal muscular atrophy: Co-existence of deletion and duplication in SMN1 gene and false negative MLPA result. Gene Rep 16:100440
Shawky RM, Abdel Aleem K, Rifaat MM, Moustafa A (2001) (2001) Molecular diagnosis of spinal muscular atrophy in Egyptians. EMHJ - East Mediterr Health J 7(1–2):229–237
Shin S, Park SS, Hwang YS, Lee KW, Chung SG, Lee YJ, Park MH (2000) Deletion of SMN and NAIP genes in Korean patients with spinal muscular atrophy. J Korean Med Sci 15(1):93–98
Sleutjes B, Wijngaarde CA, Wadman RI, Otto LAM, Asselman FL, Cuppen I, Goedee HS (2020) Assessment of motor unit loss in patients with spinal muscular atrophy. Clinical Neurophysiology : Clin Neurophysiol 131(6):1280–1286
Smeriglio P, Langard P, Querin G, Biferi MG (2020) The identification of novel biomarkers is required to improve adult SMA patient stratification, diagnosis and treatment. J per Med 10(3):75
Srivastava G, Srivastava P (2019) Spinal muscular atrophy–a revisit of the diagnosis and treatment modalities. Int J Neurosci 129(11):1103–1118
Stewart H, Wallace A, McGaughran J, Mountford R, Kingston H (1998) Molecular diagnosis of spinal muscular atrophy. Arch Dis Child 78(6):531–535
Tan CA, Westbrook MJ, Truty R, Kvitek DJ, Kennemer M, Winder TL, Shieh PB (2020) Incorporating spinal muscular atrophy analysis by next-generation sequencing into a comprehensive multigene panel for neuromuscular disorders. Gene Test Mol Biomark 24(10):616–624
Vijzelaar R, Snetselaar R, Clausen M, Mason AG, Rinsma M, Zegers M, Schouten J (2019) The frequency of SMN gene variants lacking exon 7 and 8 is highly population dependent. PLoS ONE 14(7):e0220211
Wilfinger WW, Mackey K, Chomczynski P (1997) Effect of pH and ionic strength on the spectrophotometric assessment of nucleic acid purity. Biotechniques 22(3):474–481. https://doi.org/10.2144/97223st01
Wirth B, Karakaya M, Kye MJ, Mendoza-Ferreira N (2020) Twenty-five years of spinal muscular atrophy research: from phenotype to genotype to therapy, and what comes next. Annu Rev Genomics Hum Genet 21:231–261
Wu AH (2006) Tietz clinical guide to laboratory tests-E-book. Elsevier Health Sciences, New York
Yuan P, Jiang L (2015) Clinical characteristics of three subtypes of spinal muscular atrophy in children. Brain Dev 37(5):537–541
Zhang Y, He J, Zhang Y, Li L, Tang X, Wang L, Zhang Y (2020) The analysis of the association between the copy numbers of survival motor neuron gene 2 and neuronal apoptosis inhibitory protein genes and the clinical phenotypes in 40 patients with spinal muscular atrophy: Observational study. Medicine 99(3):e18809
Acknowledgements
Not Applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Abdel Nasser H. Abd El Mutaleb, Fayed A.K. Megahed; and Ahmed Atta. The first draft of the manuscript was written by Fawziya A.R. Ibrahim and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical Approval
This study was performed in line with the principles of the Declaration of Helsinki and its later amendments or comparable ethical standards. All procedures performed and study design were reviewed and approved by the Ethical Committee in Medical Research Institute- Alexandria University.
Consent to Participate
A signed informed consent was collected from all the participants prior to enrollment in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd El Mutaleb, A.N.H., Ibrahim, F.A.R., Megahed, F.A.K. et al. NAIP Gene Deletion and SMN2 Copy Number as Molecular Tools in Predicting the Severity of Spinal Muscular Atrophy. Biochem Genet (2024). https://doi.org/10.1007/s10528-023-10657-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10528-023-10657-6