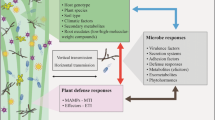
Abstract
Stripe rust, caused by Puccinia striiformis f. sp. tritici, is one of the most devastating diseases of wheat worldwide. This study dealt with investigating the biocontrol activity of mycorrhizal colonization and/or application of Streptomyces viridosporus HH1 against stripe rust of wheat. In vitro, data showed that S. viridosporus HH1 had a strong anti-spore germination effect on uredospores of P. striiformis (90% reduction). A 84.2% reduction in the disease severity was achieved in the infected wheat plants, which were colonized with mycorrhizal fungi and were sprayed with S. viridosporus HH1. Molecular investigation revealed that mycorrhizal colonization and application of S. viridosporus HH1 upregulated the defensive genes ethylene response factor protein 3 (JERF3) by 20.7-fold, chitinase II (13.6-fold), and β-1,3-glucanase (9.8-fold). Furthermore, mycorrhizal colonization and application of S. viridosporus HH1 led to a significant increase in the activity of the antioxidant enzymes peroxidase (52.3%), polyphenol oxidase (106.9%), and catalase (87.9%). The phenolic content also increased due to mycorrhizal colonization and application of S. viridosporus HH1, while the lipid peroxidation decreased in the infected wheat leaves. A mitigation in the adverse effects of infection on the photosynthetic pigments in wheat leaves was noticed. Observations from transmission electron microscopy showed that different hypersensitivity reactions were induced on the ultrastructural level in the infected wheat cells due to this treatment. In addition, a significant growth-promoting effect was also reported. It can be concluded that mycorrhizal colonization and spraying with S. viridosporus HH1 have a promising biocontrol activity against stripe rust in wheat. Field evaluation is necessary in the future studies before the use recommendation.
Similar content being viewed by others
Introduction
Wheat (Triticum aestivum L) is the most significant cereal crop worldwide with a global production of more than 7.6 billion tons produced from 2.2 billion ha in 2020 (FAOSTAT 2023). However, wheat cultivation is subjected to invasion by various pathogens, which lead to a considerable loss regarding the yield and grain quality. Stripe (yellow) rust, caused by Puccinia striiformis f. sp. tritici, is one of the most devastating diseases of wheat resulting in sever yield losses under favorable conditions (Chen 2020). In this regard, the annual global loss in wheat crop due to stripe rust is estimated at more than one billion US$ (Beddow et al. 2015). Different chemical fungicides are available to control stripe rust such as mancozeb, azoxystrobin and trifloxystrobin (Carmona et al. 2020). However, the control by chemical fungicides has many risks due to the serious effects they inflict on human and animal health, and environment. Biological control represents an eco-friendly and safe option to replace the risky chemical fungicides (He et al. 2021).
Arbuscular mycorrhizal fungi (AMF), which are obligate biotrophs that live in a mutualistic association with most terrestrial plants, have been widely studied as biocontrol agents against different pathogens (Aseel et al. 2019; Rashad et al. 2020b). In this relationship, multiple benefits are received by the host partner including enhancement of the water and nutrients acquisition, plant growth and yield, tolerance to biotic and abiotic stresses, and triggering of plant resistance against various pathogens (Montesinos-Navarro et al. 2019). Not only AMF, but also AMF together with other biocontrol agents can have beneficial effects on plants. El-Sharkawy et al. (2022) reported a synergistic biocontrol activity between Rhizophagus irregularis and Streptomyces viridosporus HH1 against Fusarium wilt of pea.
Various studies have reported the antifungal and antibacterial activities of members of the genus Streptomyces (Al-Askar et al. 2014). One of the most interesting characteristics of Streptomyces spp. is the ability to produce a wide set of secondary metabolites with different bioactivities such as antimicrobials, immunosuppressants, phytohormones, siderophores and enzymes that enables them to dominate different habitats (Rashad et al. 2017; Rashad and Moussa 2020). Chaiharn et al. (2020) found that rice plants, which were treated with S. palmae PC 12, showed improved growth parameters under the greenhouse conditions. In addition, it exhibited a strong inhibitory effect against Pyricularia sp., the causal agent of rice blast, in vitro. This study aimed to (1) investigate the biocontrol activity of colonization with AMF and/or inoculation with S. viridosporus HH1 against stripe rust of wheat under the greenhouse conditions, and (2) study effects of the applied treatments on the transcriptomic, biochemical, and ultrastructural levels, as well as on the plant growth.
Materials and methods
Used wheat cultivar and microorganisms
Wheat grains (cv. Sids-12), kindly provided by Crop Research Institute, Agricultural Research Center (ARC), Giza, Egypt, were used during the greenhouse experiment. Uredospores of P. striiformis f. sp. tritici were kindly provided by Wheat Diseases Research Department, Plant Pathology Research Institute (PPRI), ARC, Egypt. The inoculum was freshly prepared (without any incubation) by suspending the uredospores in sterile water supplemented with Tween 80 (0.1%) and gum Arabic (40 g l−1), and adjusted at 104 spore ml−1.
A pure strain of S. viridosporus HH1 was kindly provided by Bacterial Diseases Research Department, PPRI, ARC, Egypt. The bacterial inoculum was prepared by culturing S. viridosporus HH1 on potato dextrose broth (Difco, Detroit, MI, USA) at 30 °C for a week. A spore suspension (106 spore ml−1) was freshly prepared by diluting the bacterial culture using sterile water to achieve the requested concentration.
An AMF inoculum mixture (83% colonization index), kindly provided by ARC, Egypt, was used in the greenhouse experiment. The AMF inoculum contained three types of mycorrhizal fungi (Rhizophagus irregularis (Blaszk., Wubet, Renker and Buscot) Walker and Schüßler, Rhizoglomus clarum (Nicolson and Schenck) Sieverd., Silva and Oehl, and Gigaspora gigantea (Nicol. and Gerd.) Gerd. and Trappe (60 spores per gram of soil for each fungus). The inoculum consisted of fungal spore, colonized root segments, and rhizospheric soil.
Assessment of the inhibitory effect of S. viridosporus HH1 on uredospores germination in vitro
Inhibitory effect of S. viridosporus HH1 was tested on uredospores germination of P. striiformis. Fresh uredospores were spread on water agar (2%) inoculated by adding the bacterial suspension (106 CFU ml−1) in the medium before its solidification. For control treatment, agar plates which were not inoculated with the bacterial suspension were used. For each of the treated and control treatments, 150 spores per replicate were utilized. All plates were kept at 9 °C for 24 h. Germination of the uredospores was checked using a light microscope (Leica DM LB2, Leica Microsystems Wetzlar GmbH, Wetzlar, Germany). Each treatment consisted of ten replicates.
Greenhouse experiment
Pots (30 cm diameter) packed with sterile soil (sand/clay 3:1, v/v) were planted with surface-sterilized wheat grains (cv. Sids-12) at ten grains per pot. Surface sterilization of the wheat grains was performed by immersing them in sodium hypochlorite (5%) for 3 min and rinsed three times in sterile water. For half of the used pots, AMF inoculum was added under wheat grains (10 g grain−1) at planting time. Nitrogen fertilization was applied in triplicate (urea 1 g pot−1), while potassium fertilizer was applied once at the planting time (0.5 g pot−1). All pots were regularly irrigated with tap water. For inoculation with S. viridosporus HH1, wheat plants were twice sprayed with the spore suspension, 45 and 60 days after planting, until run-off occurred. Wheat plants were sprayed 60 days after planting with the chemical fungicide (Crwan® 25% EC (Propiconazole), El-Helb Pesticides & Chemicals Co, Egypt) at 0.3 ml l−1 to serve as a positive control. Seventy-two hours after the second spraying with S. viridosporus HH1, wheat plants were sprayed with the uredospores inoculum until run-off occurred. Non-infected, untreated, and nonmycorrhizal wheat plants were only sprayed with plain water to serve as a negative control. For each treatment, 15 pots were used as replicates. The pots were arranged in a complete randomized design and kept under a transparent plastic hood at 20–28 °C and 90% RH for 48 h to induce the uredospores germination, then in a greenhouse at 28/20 °C (day/night), with a 16:8 L:D photoperiod, and 60% RH. The applied treatments were as follows: nonmycorrhizal, untreated, and non-infected control (C), nonmycorrhizal, untreated, and infected (P), nonmycorrhizal, treated with fungicide, and infected (F + P), mycorrhizal, untreated, and infected (AMF + P), nonmycorrhizal, treated with S. viridosporus HH1, and infected (S + P), and mycorrhizal, treated with S. viridosporus HH1, and infected (AMF + S + P).
Biochemical analyses
Forty-eight hours post-infection, wheat leaves from each treatment were sampled for biochemical analyses. Activity of the antioxidant enzymes polyphenol oxidase (PPO), peroxidase (POD), and catalase (CAT) was estimated according to methods adopted by Galeazzi et al. (1981), Maxwell and Bateman (1967), and Chance and Maehly (1955), respectively. Total phenol content in wheat leaves was estimated according to Malik and Singh (1980). For each analysis, ten replicates were applied.
Fourteen days post-infection (dpi), the third upper leaf from each treatment was sampled and the lipid peroxidation was estimated as described by HongBo et al. (2005) and expressed as malondialdehyde (MDA) content in μmol g−1. The photosynthetic pigments was estimated according to Harborne (1984).
Transcriptional profiling of the defense-related genes
Three dpi, wheat leaves from each treatment were sampled for the molecular investigation. Total RNA was extracted from the collected samples using RNeasy Mini Kit (Qiagen, Hilden, Germany). The extracted RNA was then used to synthesize cDNA using a SureCycler 8800 (Agilent, USA). The cDNA synthesis reaction consisted of RNA (3 μl), dNTPs (2.5 μl), 5 × buffer solution (2.5 μl), RNase-free water (6.7 μl), oligo (dT) primer (5 μl), and reverse transcriptase enzyme (0.3 μl; New England Biolabs, Germany). The cDNA synthesis program was as follows: 42 °C for 1 h, 95 °C for 10 min.
The real-time PCR (qPCR) mixture consisted of cDNA (3 μl), 2xSYBR® Green RT Mix (12.5 μl; Bioloine, Germany), RNase-free water (1.5 μl), and forward and reverse primers (1.5 μl for each). Primer sequences (Rashad et al. 2021) used in this study are presented in Supplementary table S1. The qPCR reaction was done using a Rotor-Gene-6000-system (Qiagen, USA) as follows, 95 °C for 3 min, followed by 45 cycles (95 °C for 15 s, 56 °C for 30 s, and 72 °C for 30 s). The Elongation factor 1 α (EF1 α) was used as a reference gene due to its high stability in plant mycorrhizal colonization. Gene expression was measured using the comparative CT method (2−∆∆CT) (Livak and Schmittgen 2001). For each treatment, triple biological and technical replication was performed.
Ultrastructural investigation
At 7 dpi, wheat leaves were sampled for examination using a transmission electron microscope (TEM) (JEM-1230, JEOL Ltd, Tokyo, Japan). An infected leaf part (1 cm2) was gradually dehydrated using ethyl alcohol (10–100%), each for 10 min. The dehydrated sample was treated with ethanol-propylene oxide and propylene oxide-araldite, embedded in gelatin capsules, and heated at 60 °C for 60 h. Ultrathin sections were cut using Reichert Ultramicrotome and stained with uranyl acetate and lead citrate before examination.
Disease assessment
Fourteen dpi, wheat plants from each treatment were evaluated for the disease severity according to Peterson et al. (1948) using a diagrammatic scale (1 to 100%). Average coefficient of infection (ACI) was calculated by multiplying the disease severity by a constant value of the infection type (R: 0.2, MR: 0.4, MS: 0.8, and S: 1) according to Johnston and Browder (1964).
Evaluation of mycorrhization level
Fourteen dpi, ten wheat roots of different treatments were evaluated for the colonization level with AMF. Forty root segments (1 cm) of each treatment were boiled in KOH (10%), and stained using trypan blue (Sigma, St. Louis, MO, USA). Mycorrhizal colonization level was measured using light microscope. Frequency and intensity of colonization, and arbuscules frequency were estimated for each sample.
Growth evaluation
Twenty dpi, ten random plants of each treatment were carefully uprooted, washed under running water, and evaluated for plant height (cm), shoot and root dry weights (g), and leaf area (cm2). The leaf area was measured using a leaf area meter (LI-3100C, LI-COR Biosciences, Lincoln, NE, USA). Weights were determined after samples drying in an oven at 80 °C for three days.
Statistical analysis
Statistical analysis of the data was done using the software CoStat (ver 6.4). The obtained data were tested for normality, and subjected to ANOVA. Comparison of the data means was performed using Tukey's HSD test at p ≤ 0.05.
Results
Inhibitory effect of S. viridosporus HH1 on uredospores germination in vitro
Results revealed that uredospores of P. striiformis normally germinated in the control treatment. On the contrary, a strong inhibition (90%) in germination of uredospores that were treated with the bacterial suspension was recorded, compared to the control treatment, indicating the anti-spore germination activity of S. viridosporus HH1 (Supplementary figure S1).
Disease assessment
Data showed that the untreated-infected wheat plants recorded the highest disease severity (95%) and average coefficient infection (95%), compared with the control plants (Table 1). All applied treatments significantly reduced the disease severity, compared to the infected plants that were treated with the chemical fungicide. However, mycorrhizal colonization and application of S. viridosporus HH1 was more effective in this concern than the single ones, recording 84.2% reduction. No significant difference was recorded between the plants that were colonized with mycorrhizal fungi and sprayed wth S. viridosporus HH1 and that were treated with the fungicide.
Effect on activities of antioxidant enzymes, total phenol, and lipid peroxidation
The obtained results showed that all tested treatments led to an increment in the activity of POD, PPO, and CAT, compared with the control plants (Table 2). Regarding POD, no significant difference was observed between the enhancing effect due to the single treatments and that due to mycorrhizal colonization and application of S. viridosporus HH1. While, the enhancing effect on PPO and CAT due to mycorrhizal colonization and application of S. viridosporus HH1 was higher than that due to the single treatments, recording the highest values 16.9 and 34.8 unit min−1 g−1 fresh weight, respectively. However, the enhancing effect on PPO and CAT due to S. viridosporus HH1 was higher than that due to the mycorrhizal colonization. In addition, the phenolic content in wheat leaves was improved due to all applied treatments, compared with the control plants. However, the inducing effect due to mycorrhizal colonization and application of S. viridosporus HH1 was higher than that due to the single ones. Furthermore, the mycorrhizal colonization had a more enhancing effect in this case than S. viridosporus HH1. Lipid peroxidation in wheat leaves was significantly increased due to the infection with stripe rust, compared with the control plants. All tested treatments considerably lowered the lipid peroxidation in wheat leaves. No significant difference was recorded between the infected plants, which were colonized by AMF and that were sprayed with S. viridosporus HH1 (Table 2).
Transcriptional profiling of the defense-related genes
Transcriptional profiling of the responsive factor JERF3, which regulates many defense-related genes in the plant, and the antifungal genes CHI II and GLU was studied. Regarding JERF3, qPCR results indicated that all applied treatments significantly induced its expression, at varying extents, compared with the control plants (Fig. 1). In this concern, spraying with S. viridosporus HH1 was found to be more inducer than the mycorrhizal colonization. However, the highest inducing effect was recorded for the mycorrhizal colonization and application of S. viridosporus HH1 treatment recording 20.7-fold. For CHI II, mycorrhizal colonization and/or spraying with S. viridosporus HH1 triggered the gene expression, at varying degrees, compared to the control plants. However, effect of S. viridosporus HH1 was superior than the mycorrhizal colonization in this concern. The highest expression level was observed for the mycorrhizal colonization and application of S. viridosporus HH1 treatment recording 13.6-fold. For GLU, it was found that all treatments induced the gene expression at varying degrees, compared with the control plants. Results obtained indicated that S. viridosporus HH1 triggered the gene expression higher than the mycorrhizal colonization. The highest expression value was recorded for the infected wheat plants that were colonized with the mycorrhizal fungi and sprayed with S. viridosporus HH1, recording 9.8-fold (Fig. 1).
Bar charts indicate transcriptional profiles of the defense-related genes; ethylene response factor protein 3 (JERF3), chitinase II (CHI II), and β-1,3-glucanase (GLU) in wheat leaves, infected with stripe rust, in response to mycorrhizal colonization and/or spraying with Streptomyces viridosporus HH1. C: nonmycorrhizal, untreated, and non-infected control, S + P: nonmycorrhizal, treated with S. viridosporus HH1, and infected, AMF + P: mycorrhizal, untreated, and infected, and AMF + S + P: mycorrhizal, treated with S. viridosporus HH1, and infected. For each gene, bars marked with different letters are significantly different according to Tukey's HSD test (P ≤ 0.05). For JERF3, F = 48,806.6, df = 4, 10, p ≤ 0.05. For CHI II, F = 10,072.4, df = 4, 10, p ≤ 0.05. For GLU, F = 12,450.2, df = 4, 10, p ≤ 0.05. Each value is the mean of three biological samples, each was triplicate analyzed. Bars illustrate SE
Effect on the photosynthetic pigments
Results obtained revealed that infection of wheat plants with stripe rust significantly reduced all the tested photosynthetic pigments, compared to the control plants (Table 3). All applied treatments considerably mitigated the negative effect of the infection on these pigments. However, no significant difference was detected between all applied treatments.
TEM observations
TEM obsrevations of a number of cells in a wheat leaf (untreated and infected) showed disorganized cells with ultrastructural alterations surrounded with thick cell walls. The cells contained many degenerated chloroplasts, electron-dense particles, granulated cytoplasm, and a lipid body. In addition, the infected cell exhibited a large haustorium body with two haustorial nuclei, and distinct haustorial wall (Fig. 2a). On the contrary, TEM observations of a wheat leaf from the mycorrhizal, infected plant, which was sprayed with S. viridosporus HH1 showed well-organized cells surrounded with very thick cell walls and plasma membranes, containing condensed chloroplasts. Furthermore, the infected cell showed an abnormal and small haustorium body enclosed by a thin haustorial wall (Fig. 2b).
Transmission electron micrographs show ultrastructure alterations in wheat leaves infected with stripe rust in response to mycorrhizal colonization and spraying with Streptomyces viridosporus HH1. a: a leaf from nonmycorrhizal, untreated, and infected plant showing disorganized cells surrounded by thick cell walls (Tcw) and containing many degenerated chloroplasts (DC), electron-dense particles (EDp), a granulated cytoplasm (Cy) and a lipid body (LB). Note a large haustorium body with two haustorial nuclei (Hn) and distinct haustorial wall (Hw). b: a leaf from mycorrhizal, sprayed with S. viridosporus HH1, and infected plant showing well-organized cells surrounded with very thick cell walls (Tcw) and thick plasma membranes (Tpm), and contained large chloroplasts (C). Note a probable abnormal and small haustorium body enclosed by a thin haustorial wall
Effect on mycorrhization level
No mycorrhizal colonization was observed in all treatments that not received AMF. In contrast, wheat roots from the treatment (AMF + P) showed 48.7% colonization frequency, 35.1% colonization intensity and 24.2% arbuscules frequency (Supplementary table S2). No significant differences were recorded between the treatments (AMF + P) and (AMF + S + P) regarding these parameters. Mycorrhizal structures in wheat roots that were colonized with AMF are illustrated in the Supplementary figure S2.
Effect on plant growth
The results showed that infection of wheat plants led to a significant reduction in the shoot height, and shoot and root dry weights, while the leaf area was not affected, compared with the control plants (Table 4). In addition, all tested treatments considerably mitigated the adverse effect, which resulted due to the infection, compared to the infected, untreated plants. Mycorrhizal colonization and/or spraying with S. viridosporus HH1 significantly improved the leaf area in the infected plants, compared with the untreated, infected plants.
Discussion
Stripe rust is one of the most devastating diseases of wheat worldwide (Chen 2020). The anti-spore germination effect of S. viridosporus HH1 against uredospores of P. striiformis was studied in vitro. Results obtained showed that S. viridosporus HH1 had a strong inhibitory effect on the uredospores germination (90% inhibition). Uredospore of P. striiformis has a fast germination rate. It needs just 3 h to initiate its germination towards the stomata, and additional 8–18 h to form the appressorium, penetrate the stomatal cavity, develop between the mesophyll cells, and penetrate them by forming haustoria (Chen et al. 2014). Although its fast development before the leaf penetration, this stage represents a good chance to inhibit the infection progress of P. striiformis. Results from a previous study constructed by El-Sharkawy et al. (2022) on S. viridosporus HH1 confirmed its antifungal potential against F. oxysporum via production of different antifungal secondary metabolites including 2,3-butanediol, thioglycolic acid, and phthalic acid. These antifungal compounds may explain the anti-spore germination effect against uredospores of P. striiformis, representing their crucial role in reducing the infection of wheat plants.
Results in this study indicated a significant biocontrol potential for colonization of wheat roots with AMF and spraying its leaves with S. viridosporus HH1, which led to a significant reduction in the disease severity. Biological control using AMF has been widely studied against different plant diseases (Aseel et al. 2019; El-Sharkawy et al. 2022; Rashad et al. 2022). In this regard, El-Sharkawy et al. (2018) found that mycorrhizal colonization and treating with Trichoderma harzianum HL1 and T. viride HL5 considerably reduced the disease measures and triggered the defensive responses in wheat plants against stem rust. It has been reported that once mycorrhizal colonization is established in the host roots, a genome-wide reprogramming is occurred resulting in modulations in the plant responses to different biotic and abiotic stresses (Stratton et al. 2022). TEM observations in this study exhibited a cell wall thickening in the infected plants, which were colonized with the AMF. It seemed that the cellular lignification played an important role in blocking the penetration of the haustoria of P. striiformis to the wheat cells by enhancing their rigidity, as well as preventing water and nutrients transport from the cell to the haustorium. This result is in agreement with that reported by Miedes et al. (2014). Results from this study indicated an elevation in the phenolic content in the infected leaves of the mycorrhizal wheat plants. The antifungal modes of action of the polyphenolic compounds include changing permeability of the cell membrane, inactivation of the functional enzymes, inducing oxidative bursts, and/or suppression of important proteins (Rashad et al. 2020a). Results obtained in this study indicated that mycorrhizal colonization and application of S. viridosporus HH1 led to a considerable activation for POD, PPO, and CAT, as well as a reduction in the lipid peroxidation. These antioxidant enzymes act as scavengers for the reactive oxygen species (ROS) and free radicals that result due to the infection and lead to destruction of the cell components and finally the cell death.
Furthermore, mycorrhizal colonization has been reported to induce the transcriptional expression level of various defense-related genes in the plants against different biotic stresses (Rashad et al. 2021). In this regard, the obtained results showed that mycorrhizal colonization of wheat plants and application of S. viridosporus HH1 triggered expression level of the defense-related genes JERF3, CHI II, and GLU. JERF3 is a responsive factor that activates numerous defensive genes via the jasmonate (JA) and ethylene (ET) pathways against different biotic and abiotic stresses (Rashad et al. 2022). In the line with this result, qPCR analysis from this study indicated that upregulation of JERF3 was found to be associated with induction of CHI II and GLU. CHI II encodes for the antifungal enzyme chitinase, which cleaves the glycosidic bonds in the chitin molecule, the main subunit in the fungal cell wall, leading to damaging of the fungal cells (Malik and Preety 2019), while GLU encodes for the fungal cell-wall lytic enzyme β-1,3-glucanase that acts on hydrolysis of the glucan molecule, which forms with the chitin molecule the main backbone in the fungal cell wall (Balasubramanian et al. 2012). Destruction of the fungal cell walls, haustoria of P. striiformis in this case, represents a crucial resistance mechanism that may contributed in inhibition of P. striiformis. In general, plant responses to the applied microorganisms represent the net result of the crosstalks between different induced phytohormone signaling pathways, namely JA, ET, salicylic acid (SA), and/or abscisic acid (ABA). These signaling pathways are synergistically or antagonistically interconnected to form a resistance network in which numerous defensive mechanisms are involved (Liu and Lam 2019). Various members of the genus Streptomyces have been reported as plant resistance inducers (Abbasi et al. 2019). In this regard, El-Sharkawy et al. (2022) found that resistance of pea plants were induced against Fusarium wilt when were sprayed with of S. viridosporus HH1. Furthermore, they reported the production of 2,3-butanediol by S. viridosporus HH1 as one of its secondary metabolites. This volatile substance has been reported as a plant-immunity elicitor, through ET signaling pathway, against numerous biotic stresses (Park et al. 2018). Production of 2,3-butanediol by S. viridosporus HH1 may explain its role in induction of wheat immunity against stripe rust.
In addition, results obtained in this study revealed that the applied treatments showed a growth-promoting effect. Numerous mechanisms have been discussed in this concern including improving absorption of water and nutrients via the extraradical mycelial network, secretion of variable organic acids, hydrolyzing enzymes such as phosphatases, and siderophores in the host rhizosphere (Andrino et al. 2021). Synthesis of variable phytohormones such as cytokinins, auxins, and gibberellins has been also reported (Zou et al. 2017). All or most of these mechanisms may synergistically contributed in the growth-promoting effect, which was reported in this study for AMF.
Plant-growth promotion by Streptomyces spp. has also been reported on different crops (Olanrewaju and Babalola 2019). Different mechanisms have been recognized for the growth-promoting activity of Streptomyces spp. including enhancing nutrients acquisition, improving photosynthesis pigments content and efficiency, and synthesis of various phytohormones and growth promoters (Amaresan et al. 2018). Production of the volatile compound 2,3-butandiol by S. viridosporus HH1 (El-Sharkawy et al. 2022) may explain the growth enhancement reported in this study due to S. viridosporus HH1.
In conclusion, this study dealt with investigating the biocontrol activity of mycorrhizal colonization and/or application of S. viridosporus HH1 against stripe rust of wheat. In vitro, S. viridosporus HH1 showed an inhibitory effect on germination of P. striiformis uredospores confirming its direct antifungal mode of action. Results achieved from the greenhouse experiment showed a reduction in the disease severity in response to mycorrhizal colonization and application of S. viridosporus HH1. Furthermore, this treatment triggered transcriptional expression of the defense related genes JERF3, CHI II and GLU. Activity of POD, PPO and CAT, and phenolic content increased due to this treatment. While the lipid peroxidation level reduced. Different hypersensitivity reactions were observed at the ultrastructural level in the infected wheat cells due to this treatment. Moreover, mycorrhizal colonization and treating with S. viridosporus HH1 exhibited a significant growth-promoting effect. Based on the achieved results, it can be concluded that mycorrhizal colonization and spraying with S. viridosporus HH1 have a promising biocontrol activity against stripe rust in wheat. Field evaluation of this treatment is necessary in the future studies before its use recommendation.
References
Abbasi S, Safaie N, Sadeghi A, Shamsbakhsh M (2019) Streptomyces strains induce resistance to Fusarium oxysporum f sp lycopersici race 3 in tomato through different molecular mechanisms. Front Microbiol 10:1505
Al-Askar AA, Abdulkhair WM, Rashad YM, Hafez EE, Ghoneem KKM, Baka ZA (2014) Streptomyces griseorubens E44G: A potent antagonist isolated from soil in Saudi Arabia. J Pure Appl Microbiol 8:221–230
Amaresan N, Kumar K, Naik JH, Bapatla KG, Mishra RK (2018) Streptomyces in plant growth promotion: mechanisms and role. In: Singh BP and Passari A (eds) New and future developments in microbial biotechnology and bioengineering. Actinobacteria: diversity and biotechnological applications. Elsevier, USA
Andrino A, Guggenberger G, Kernchen S, Mikutta R, Sauheitl L, Boy J (2021) Production of organic acids by arbuscular mycorrhizal fungi and their contribution in the mobilization of phosphorus bound to iron oxides. Front Plant Sci 12:661842
Aseel DG, Rashad YM, Hammad SM (2019) Arbuscular mycorrhizal fungi trigger transcriptional expression of flavonoid and chlorogenic acid biosynthetic pathways genes in tomato against Tomato Mosaic Virus. Sci Rep 9:9692
Balasubramanian V, Vashisht D, Cletus J, Sakthivel N (2012) Plant β-1,3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol Lett 34:1983–1990
Beddow JM, Pardey PG, Chai Y, Hurley TM, Kriticos DJ, Braun HJ, Park RF, Cuddy WS, Yonow T (2015) Research investment implications of shifts in the global geography of wheat stripe rust. Nat Plants 1:15132
Carmona M, Sautua F, Pérez-Hérnandez O, Reis EM (2020) Role of fungicide applications on the integrated management of wheat stripe rust. Front Plant Sci 11:733
Chaiharn M, Theantana T, Pathom-Aree W (2020) Evaluation of biocontrol activities of Streptomyces spp against rice blast disease fungi. Pathogens 9(2):126
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. In: Chance B (ed) Methods in enzymology. Academic Press, USA
Chen X (2020) Pathogens which threaten food security: Puccinia striiformis, the wheat stripe rust pathogen. Food Secur 12:239–251
Chen W, Wellings C, Chen X, Kang Z, Liu T (2014) Wheat stripe (yellow) rust caused by Puccinia striiformis f. sp. tritici. Mol Plant Pathol 15:433–446
El-Sharkawy HHA, Rashad YM, Elazab NT (2022) Synergism between Streptomyces viridosporus HH1 and Rhizophagus irregularis effectively induces defense responses to Fusarium wilt of pea and improves plant growth and yield. J Fungi 8(7):683
El-Sharkawy HHA, Rashad YM, Ibrahim SA (2018) Biocontrol of stem rust disease of wheat using arbuscular mycorrhizal fungi and Trichoderma spp Physiol Mol Plant Pathol 103:84–91
FAOSTAT (2023) Food and agriculture organization of the United Nations. http://www.fao.org/faostat/en/#data/QC
Galeazzi MAM, Sgarbieri VC, Constantinides SM (1981) Isolation, purification and physicochemical characterization of polyphenoloxidases (PPO) from a dwarf variety of banana (Musa cavendishii, L). J Food Sci 46:150–155
Harborne JB (1984) Phytochemical methods: A guide to modern techniques of plant analysis. Chapman and Hall, London
He DC, He MH, Amalin DM, Liu W, Alvindia DG, Zhan J (2021) Biological control of plant diseases: An evolutionary and eco-economic consideration. Pathogens 10(10):1311
HongBo S, ZongSuo L, MingAn S (2005) Changes of anti-oxidative enzymes and MDA content under soil water deficits among 10 wheat (Triticum aestivum L.) genotypes at maturation stage. Colloids Surf B Biointerf 45:7–13
Johnston CO, Browder LE (1964) Seventh revision of the international register of physiologic races of Puccinia recondita f. sp. tritici. Plant Dis Report 50:756–760
Liu JZ, Lam HM (2019) Signal transduction pathways in plants for resistance against pathogens. Int J Mol Sci 20(9):2335
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408
Malik A, Preety, (2019) Purification and properties of plant chitinases: A review. J Food Biochem 43:e12762
Malik CP, Singh MB (1980) Estimation of total phenols. In: Malik C (ed) Plant enzymology and histo-enzymology. Kalyani Publishers, New Delhi, India
Maxwell DP, Bateman DF (1967) Changes in the activities of some oxidases in extracts of Rhizoctonia-infected bean hypocotyls in relation to lesion maturation. Phytopathology 57:132–136
Miedes E, Vanholme R, Boerjan W, Molina A (2014) The role of the secondary cell wall in plant resistance to pathogens. Front Plant Sci 5:358
Montesinos-Navarro A, Valiente-Banuet A, Verdú M (2019) Mycorrhizal symbiosis increases the benefits of plant facilitative interactions. Ecography (cop) 42:447–455
Olanrewaju OS, Babalola OO (2019) Streptomyces: implications and interactions in plant growth promotion. Appl Microbiol Biotechnol 103:1179–1188
Park KY, Seo SY, Oh BR, Seo JW, Kim YJ (2018) 2,3-butanediol induces systemic acquired resistance in the plant immune response. J Plant Biol 61:424–434
Peterson RF, Campbell AB, Hannah AE (1948) A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can J Res 26:496–500
Rashad YM, Moussa TAA (2020) Biocontrol agents for fungal plant diseases management. In: El-Wakeil N, Saleh M, Abu-hashim M (eds) Cottage industry of biocontrol agents and their applications: Practical aspects to deal biologically with pests and stresses facing strategic crops. Springer International Publishing, Cham, pp 337–363
Rashad YM, Al-Askar AA, Ghoneem KM, Saber W, Hafez EE (2017) Chitinolytic Streptomyces griseorubens E44G enhances the biocontrol efficacy against Fusarium wilt disease of tomato. Phytoparasitica 45:227–237
Rashad Y, Aseel D, Hammad S (2020a) Phenolic compounds against fungal and viral plant diseases. In: Lone R (ed) Plant phenolics in sustainable agriculture. Springer Singapore, Singapore
Rashad Y, Aseel D, Hammad S, Elkelish A (2020b) Rhizophagus irregularis and Rhizoctonia solani differentially elicit systemic transcriptional expression of polyphenol biosynthetic pathways genes in sunflower. Biomolecules 10:379
Rashad YM, Fekry WME, Sleem MM, Elazab NT (2021) Effects of mycorrhizal colonization on transcriptional expression of the responsive factor JERF3 and stress-responsive genes in banana plantlets in response to combined biotic and abiotic stresses. Front Plant Sci 12:742628
Rashad YM, El-Sharkawy HHA, Elazab NT (2022) Ascophyllum nodosum extract and mycorrhizal colonization synergistically trigger immune responses in pea plants against Rhizoctonia root rot, and enhance plant growth and productivity. J Fungi 8(3):268
Stratton CA, Ray S, Bradley BA, Kaye JP, Ali JG, Murrell EG (2022) Nutrition vs association: Plant defenses are altered by arbuscular mycorrhizal fungi association not by nutritional provisioning alone. BMC Plant Biol 22:400
Zou YN, Wang P, Liu CY, Ni Q-D, Zhang D-J, Wu Q-S (2017) Mycorrhizal trifoliate orange has greater root adaptation of morphology and phytohormones in response to drought stress. Sci Rep 7:41134
Acknowledgements
Authors are grateful to Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB) for providing the open access fees.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial or non-financial interests.
Research involving human and/or animal rights
This article does not contain any studies with human participants or animals (vertebrates) performed by any of the authors.
Additional information
Handling Editor: Sotiris Tjamos.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Sharkawy, H.H.A., Rashad, Y.M. & Elazab, N.T. Induction of multiple defense responses in wheat plants against stripe rust using mycorrhizal fungi and Streptomyces viridosporus HH1. BioControl 68, 525–535 (2023). https://doi.org/10.1007/s10526-023-10207-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-023-10207-4