Abstract
Some species of ladybird beetles (Coleoptera: Coccinellidae) mate both before and after overwintering. The purpose of the pre-diapause mating was studied in the alien invasive ladybird Harmonia axyridis (Pallas 1773). Our study demonstrates the persistence of high fecundity (daily oviposition rate of 21 eggs per fertilized female during the first month of reproduction) and fertility (85 % of eggs hatching) of females of H. axyridis after long storage (up to eight months) at low temperature (6 °C). The females were not mated after activation in spring and had to rely on the sperm supply maintained from the pre-winter period (58 % of females were fertilized). Unfertilized females also laid eggs but in low numbers (an average of 345 eggs by virgin females during an individual’s lifetime, 1,174 eggs by females fertilized before winter) and after a longer pre-oviposition period (2–5 weeks in comparison to 7–8 days for fertilized females). We show that the unfertilised eggs were not trophic eggs. The high sperm survival ability observed questions the need for the high levels of sexual activity generally observed in Coccinellidae. Fertilized females of H. axyridis may found large colonies after dispersal to new areas even without males, which contributes to the striking invasive ability of this species.
Similar content being viewed by others
Introduction
The life cycle of ladybird beetles (Coleoptera: Coccinellidae) in temperate regions includes one or more generations of development from egg to adult during spring and summer (Nedvěd and Honěk 2012) and adult diapause during winter. This adaptive state has two main functions: (1) to synchronize the occurrence of feeding stages with favourable seasons by preventing reproduction, (2) to increase the chance of survival during the harsh season by lowering metabolic rate, and accumulating energetic reserves and substances increasing cold hardiness (Hodek 2012).
Adult diapause is the most common form of diapause in Coleoptera, occurring in about 90 % of beetle species (Danks 1987) including most Coccinellidae (Hodek 2012). Most species select their winter quarters in September or early October. The short day conditions in late summer prevent reproduction, and induce accumulation of fat reserves (Hodek and Cerkasov 1961) and seeking of overwintering sites.
Harmonia axyridis (Pallas 1773) (Coleoptera: Coccinellidae) is a ladybird beetle native to east Asia and established in several continents as an invasive alien species (Brown et al. 2011). In warmer parts of its native range, it undergoes both a weak warm-season aestivation and a cold-season diapause (Sakurai et al. 1992). The invasive population in Europe has two to three generations and only a weak winter diapause. The effects of day length on reproduction have been investigated using a series of laboratory trials. The rate of reproductive maturation of females in the Far East Russian population is twice as high under long day length conditions (18 h light (L), 6 h dark (D) per day) than under short day length conditions (10L:14D) but most females start to reproduce even under short day lengths (Reznik and Vaghina 2011). Oviposition of the European invasive population from Belgium is delayed by 1–3 months when reared at 12L:12D in comparison with 16L:8D (Berkvens et al. 2008). Pre-oviposition period is short throughout the winter in the Netherlands, indicating that H. axyridis does not enter diapause but remains in a quiescent state (Raak-van den Berg et al. 2012a).
Migration of H. axyridis to dormancy sites occurs in autumn during warm, calm days (Obata et al. 1986; Sakurai et al. 1993; Wang et al. 2011). In spring (in late March to late April), the beetles disperse slowly from their hibernation sites toward their feeding and breeding sites (Obata et al. 1986; Osawa 2001).
In Asia, H. axyridis overwinters in rock crevices or in buildings, and sometimes in leaf litter (Obata et al. 1986; Sakurai et al. 1993). In Japan, the second or the third generation of H. axyridis aggregates at overwintering sites in early November, and adults enter reproductive diapause before winter, decrease their respiration rate, accumulate glycogen and lipids (Sakurai et al. 1992), and synthesize a diapause specific haemolymph protein. After spontaneous reactivation or artificial juvenile hormone analogue application, haemolymph protein specific to reproductive females is synthesized and the diapause protein disappears. The protein content of the yolk is usually approximately equal to the lipid content, and 60–90 % of yolk proteins are derived from vitellogenins (Okuda and Chinzey 1988; Sakurai et al. 1987).
Diapause of males of ladybirds is usually weaker than in females: males may keep active testicular follicles despite otherwise showing signs of dormancy (Hodek and Ceryngier 2000). Mating thus may occur in ladybirds both before entering overwintering shelters and after activation in spring. Most insects store sperm internally in the spermatheca, often for prolonged periods of time. Differential sperm storage between diapausing and non-diapausing ladybird females has been examined in a few ladybird species. While part of overwintering females of H. axyridis and Coccinella septempunctata L. had sperm in their spermathecae, in Ceratomegilla undecimnotata (Schneider) no spermatheca contained any sperm (Ceryngier et al. 2004). This sperm can be used for egg fertilization after the end of diapause and eliminate the need for females to copulate in spring to start reproduction. High rates of sperm transfer and a high level of mating success across a population potentially increase the re-invasion of feeding habitats after each winter and the abundance of the next generation.
Multiple mating in ladybirds may either increase or decrease total fecundity of a female. Both the number of eggs laid (fecundity) and the proportion of hatched eggs (hatching rate) increases with increasing number or frequency of mating (Bind 2007; Omkar and Mishra 2005b; Omkar and Pervez 2005a). However, hatching rate is high in several ladybird species even when females mated only once (Omkar et al. 2005). In a previous study (unpublished) we found even higher fecundity and fertility in females of H. axyridis that were mated only in autumn before overwintering than in females that were regularly mated also after overwintering, because the latter had shorter longevity.
Ability to survive prolonged cold storage with retaining reproductive capacity is an important property of many biocontrol agents (Gardner et al. 2012). Raak-van den Berg et al. (2012b) found high winter survival rate of H. axyridis mainly in sheltered sites. Ruan et al. (2012) studied the effect of cold storage on field samples of H. axyridis for 30, 60, 90, 120 and 150 days at −3, 0, 3 and 6 °C in terms of survival, fecundity and predation. Survival rapidly decreased at the two lower temperatures, while it was high at the two higher temperatures even after five months. Cold storage shortened pre-oviposition period and did not reduce reproductive performance of the ladybirds.
The three objectives of this study were (i) to demonstrate whether the sperm in ladybird females fertilized in autumn can effectively survive to spring after prolonged cold exposure (hatching rate), (ii) to investigate whether a single fertilized female may then establish a new large colony, which would partially explain the invasive success of the species studied (fecundity), and (iii) to assess the potential application of ladybirds as biocontrol agents after long storage (changes in time of fecundity, fertility and protein content). To achieve these objectives, we measured the reproductive condition of females of the European invasive population of H. axyridis after prolonged cold storage during winter by recording their fecundity after their return to suitable conditions. We also measured body soluble protein content and characterized the main protein type changes during overwintering. Furthermore, we wanted to record the level of vitality of sperm after the onset of reproduction when females were deprived of any further mating. We measured this vitality as egg hatching rate.
Materials and methods
Collecting and storage of beetles
In October 2010, migrating adults of H. axyridis were collected in České Budĕjovice, South Bohemia, Czech Republic. Beetles were transported to the Faculty of Science at the University of South Bohemia in jars at low temperature to prevent mating. They were stored in groups of 20, with sexes separated (females distinguished by a black spot on clypeus), in Petri dishes of 9 cm in diameter at low temperature (6 ± 1 °C) and continuous darkness. We chose the temperature that is both suitable for the beetle survival and technically easy to achieve in practical use. The dishes were lined with wet filter paper. Each month, the paper was renewed and the adults were allowed to spend 2 h at 20 °C to recover from part of cumulative chill injury (Hanč and Nedvěd 1999) which enabled most individuals to survive until June.
Sperm survival experiment
We took a sample of 20 adult quiescent females of the morph succinea (see Tan 1946) only (to avoid potential differences in reproductive parameters in the melanics) from the stock in cold storage each month from January to June (except in May). This morph represents about 86 % of H. axyridis adults in České Budĕjovice (Nedvědová et al. 2013). Females were weighed using electronic balances and maintained individually in Petri dishes (9 cm diameter) with wrinkled filter paper, a vial with water and fed daily with pea aphid Acyrthosiphon pisum (Harris) under long day length conditions (18 h light: 6 h dark) at 20 °C. Eggs were removed daily, counted, and kept under the same conditions until hatching or until one week had passed if no larvae hatched. We counted the number of new born larvae, number of unhatched grey eggs with embryo and number of yellow non-developing eggs. The pre-oviposition period, percentage of fertilized females, fecundity (number of eggs laid), fertility (number of larvae hatched), and hatchability rate (%) were recorded for each female.
Statistical analyses
One-way and two-way ANOVA was used for comparison of normally distributed continuous variables (fecundity and body mass) with factors being fertilized versus non-fertilized females, and month of transfer of sample from cold storage. Tukey HSD post-hoc tests were used for comparisons between individual treatments. The relationship between fecundity and body mass was analysed using ANCOVA. Hatching rate, measured as a proportion, was analysed using a non-parametric Kruskal–Wallis test. All tests were performed in Statistica 10 software (StatSoft 2011).
Protein quantification
To determine the amount of all proteins in the body remaining after long storage without access to food, we used standard spectrophotometric methods (Ornstein 1969). A sample of twenty females was taken each month from January through June from the stock of beetles stored at 6 °C. Individuals were weighed on electronic balances and then frozen at −20 °C. Individual beetles were homogenized in Eppendorf tubes using a polypropylene pestle and the soluble proteins extracted in Ringer buffer containing 0.65 % NaCl, 0.014 % KCl, 0.012 % CaCl2, and 0.1 % NaHCO3. Each sample was vortexed, centrifuged, the supernatant separated, the pellet extracted by the same procedure for the second time, and the supernatants merged. Total protein was determined with protein quantification kit-rapid based on the Coomassie brilliant blue G, measuring the absorbance of the solution at 600 nm using a spectrophotometer. Bovine serum albumin was used as a standard.
Protein characterization
Part of the above prepared extract was fractionated by SDS-PAGE according to Laemmli (1970). Electrophoresis was performed in NuPAGE® 4–12 % Bis–Tris mini gel (10 cm × 10 cm) (Invitrogen). Proteins were detected by staining with Coomassie brilliant blue R-250, and compared with a protein ladder (Thermo Scientific) of 250 kDa.
After SDS-PAGE, the separated proteins were electroblotted onto nitrocellulose foil (0.1 μm pore). Five per cent skim milk in phosphate-buffered saline containing 0.5 % Tween 20 (PBS-Tween) was used to saturate non-specific binding sites. Nitrocellulose sheets were then overlaid with the first antibody solution (dilution 1:500 in PBS-Tween) and incubated for 1 h at 4 °C. We used antibody against vitellogenin derived from Pyrrhocoris apterus (L.), assuming that the domain is phylogenetically conserved. Antigen–antibody complexes were visualized by means of another reaction with the second antibody goat anti-rabbit IgG-HRP diluted 1:1,000 in PBS-Tween, kept for 30 min at room temperature. Vitellogenin was detected by chemoluminescence using Amersham ECL direct nucleic acid labeling and detection system (Socha et al. 1991).
Results
Proportion of fertilized females
We found that overall 58 % of the females laid at least some eggs that developed into larvae, which indicates that they were fertilized before overwintering. A small number of females did not lay any eggs. Most unfertile females laid at least a small number of eggs that remained undeveloped, as indicated by the retained yellow colour. Fertilized females were heavier than virgin females in the April sample (F 1,16 = 6.84, p = 0.019) but not in other months (F 1,90 = 0.217, p = 0.647).
Pre-oviposition period
Females transferred from cold storage to rearing conditions in January, February, March, April and June needed on average 17.2, 9.6, 9.3, 11.7 and 13.5 days, respectively, to start laying eggs. Since the distribution of individual values was strongly skewed, median values were lower: 11, 8, 7, 9 and 15 days. If only the fertilized females were considered, the mean pre-oviposition periods were much shorter: 8.5, 7.1, 6.7, 8.5, 12.9, medians were 8.5, 7, 7, 8, 15 (close to the means due to the symmetrical distribution of values).
The difference in mean pre-oviposition period between fertilized and virgin females was strongly significant (shorter in fertilized females; ANOVA: F 1,90 = 21.86, p < 0.001), while the differences between months were not (F 4,90 = 1.63, p = 0.17) and there was no interaction between the two factors (F 4,90 = 1.45, p = 0.22). If only the fertilized females were considered, the difference between months becomes significant (ANOVA: F 4,52 = 7.58, p < 0.001). The period for the June sample was significantly longer than for the February to April samples (Tukey HSD post-hoc test). Body mass did not influence significantly the pre-oviposition period (ANCOVA with mass as covariate, month and fertilization status as categorical predictors: F 1,52 = 0.17, p = 0.682).
Fecundity
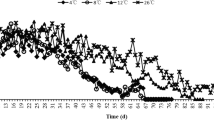
Fertilized females transferred from the cold storage to the laboratory in January laid 21.2 eggs per day per female during the first month (30 days period from the oviposition of the first female). In the subsequent months the mean egg production decreased. Females activated later showed similar initial high values and a gradual decrease of fecundity (Fig. 1). In total, we observed 82,573 eggs of H. axyridis in this study. The maximum number of eggs laid within one day by one female was found in the March sample and was 86. Maximum observed lifetime fecundity was that of one female in the January sample, which was 2,497 eggs.
Gradual decrease in fecundity (number of eggs per day per fertilised female) of females of Harmonia axyridis, morph succinea, activated after overwintering in January (circles, solid line), February (squares, dotted line), March (triangles, solid line), April (crosses, dotted line) and June (stars, combined line)
Fertility during the first month was lower in the June sample (F 4,137 = 4.32, p = 0.0025; with Tukey HSD post-hoc test p = 0.002 to 0.031) while the fecundity did not differ between January through April samples (p = 0.953 to 0.999).
The mean lifetime fecundity of fertilized females of H. axyridis was 1,174 eggs, and of virgin females (including those not ovipositing) 345 eggs, the difference being highly significant (two-way ANOVA: F 1,90 = 141.25, p < 0.001). With increasing time in cold storage, mean lifetime fecundity decreased (two-way ANOVA: F 4,90 = 4.40, p = 0.0027) in both fertilized and virgin females without interaction (F 4,90 = 0.42, p = 0.79). Within fertilized females, June samples had lower lifetime fecundity (928 eggs) than January samples (1,517 eggs) and March samples (1,385 eggs) (Tukey HSD tests: p = 0.019, 0.013). Lifetime fecundity was higher in heavier fertilized females (analyzed with the time of storage by ANCOVA: F 1,46 = 14.34, p < 0.001). Fecundity was not correlated with pre-oviposition period (analyzed with the time of storage by ANCOVA: F 1,46 = 1.41, p = 0.242).
Hatching rate
Females of H. axyridis showed a high capacity to maintain vital sperm. Females transferred from cold storage to the laboratory in January had a high percentage of hatchability in the first month of reproduction (82 %) and in the subsequent months the hatching rate gradually decreased (Fig. 2). The samples activated later showed similar initial high values and a gradual decrease of fertility. Hatching rate within the first month did not decrease from the January to the June sample (χ2 4 = 6.98, p = 0.137).
Protein concentration
Females collected outdoors in October during migration had 4.5 % of soluble proteins in their bodies (fresh weight). This concentration significantly decreased after one month of cold storage (November: 3.8 %, Tukey test following one way ANOVA: p = 0.003) and then gradually decreased further through May (2.6 %) (ANOVA: F 8,171 = 27.02, p < 0.00001, Fig. 3) and remained stable till June (2.6 %).
Gradual decrease of soluble protein concentration (‰) in females of Harmonia axyridis, morph succinea, after sampling (October = 0) and after cold storage for one to eight months (November through June). Significantly different values are marked with different letters and vertical bars denote 0.95 confidence intervals
Protein types
Gel electrophoresis showed the dynamics of major proteins during the long term cold storage (Fig. 4). Concentration and number of types of protein were high at the beginning and the end of the period, and there was no permanent gradual decrease of concentration and number of proteins. A protein with molecular weight >250 kDa, which may represent vitellogenin, was present in the SDS-PAGE gel. Vitellogenin was detected by subsequent western blotting in small amounts in October and November, disappeared in December and increased in February through June (Fig. 5).
Seasonal changes in total soluble protein profile from females of Harmonia axyridis, morph succinea, in samples from October (A) through June (I) visualized by gel electrophoresis = sodium dodecyl sulphate-PAGE. Each lane was loaded with a sample containing 10 μg of polypeptides. M protein ladder with molecular mass indicated
Discussion
As there is growing interest in the reproductive biology of ladybird beetles, we studied fecundity, hatching rate and thus sperm survival in the overwintering females of invasive species H. axyridis after cold storage lasting several months.
Female fertilization
Percentage of females fertilized before entering winter dormancy, which we estimated as females that laid eggs developing to the larval stage, was 58 % and varied between 30 % and 80 % in individual subsamples, which is comparable to our previous study (20–60 %) that used dissection and direct observation of the content of spermathecae (Nedvědová et al. 2013). Iperti and Bertand (2001) found full spermathecae in 37, 10, 53 and 17 % of overwintering females of H. axyridis at four sites in France. This population was released in southeastern France earlier and separately from the current invasive population distributed in most of Europe. However, the proportion of full spermathecae reported for H. axyridis from the USA was smaller (12 %; Nalepa et al. 1996).
The higher proportions of fertilized H. axyridis females are similar to the values found in C. septempunctata (28–47 %; Ceryngier et al. 1992). In Coccinella quinquepunctata L., the proportion of fertilized females was low (10 %) in September, but high (80 %) in January (Hodek and Ceryngier 2000), indicating that females mated at the overwintering sites. While most overwintering females of C. septempunctata and C. quinquepunctata belong to the new generation that mated but did not lay eggs before overwintering, many females of H. axyridis that migrate to the overwintering sites seem to belong to the early summer generation (they appear older according to their deep red elytral colour—unpublished observation) and have already reproduced. Sperm stored in the spermatheca of these females may therefore be a few months old even at the date of sampling.
Ladybird females can conserve the sperm for a long time in their spermathecae probably thanks to the nutritive compounds produced by accessory glands, including several proteins and a carbohydrate-protein complex (Gillott 1988). This could explain why the overwintering females of H. axyridis have a high capacity to maintain vital sperm for such a long time even after termination of their dormancy.
The females of many ladybirds need not copulate in spring to start reproduction soon after winter dormancy. However, in some species such as C. undecimnotata, no females were found to store sperm during dormancy (Hodek and Ceryngier 2000) and only a few diapausing females of Adalia bipunctata (L.) were reported to contain sperm (Arnaud et al. 2001). It is notable that most unfertilized females of H. axyridis also laid some eggs after transfer to suitable conditions, but the pre-oviposition period of these virgin females was much longer and fecundity much smaller than in fertilized females.
Pre-oviposition period
The time lapse between the transfer to suitable rearing conditions (temperature, photoperiod, food) and laying their first egg clutch is usually used as an indicator of diapause intensity in insects (Hodek 2012). It usually decreases substantially during the winter. H. axyridis has a shallow diapause and spends a great part of its dormancy in a quiescent state. Females transferred to rearing conditions in January already had a very short pre-oviposition period. This also applies to females activated in October to November (unpublished data). This corresponds to the high content of proteins and presence of vitellogenin in those females during cold storage (Figs. 4, 5).
Pre-oviposition periods as short as seven or eight days measured in our samples from January to April are similar to those measured in a population introduced to southeastern France (Iperti and Bertand 2001) in February (nine days) and March (seven days) and indicate that all females were in postdiapause quiescence. Earlier French samples (November, December, January) had only slightly longer periods (11, 16, 14 days). Pre-oviposition periods have been found to be short throughout winter in the Netherlands, which might indicate that H. axyridis was not in diapause but in a quiescent state (Raak-van den Berg et al. 2012a). However, pre-oviposition period in the population in the Netherlands gradually decreased from ten to five days from December to March.
A similarly short pre-oviposition period has been found in non-diapausing females of other species. Females of A. bipunctata reared at long photoperiod had 8–10 day pre-oviposition periods, but the pre-oviposition period of this species transferred into the laboratory from the field substantially decreased from 11 days in December to seven days in March and three days in May (Obrycki et al. 1983).
The duration of the pre-oviposition period decreased after prolonged cold storage (up to five months) at both 3 °C and 6 °C and 90 day storage at 0 °C resulted in the shortest pre-oviposition period in a Chinese population (Ruan et al. 2012). The pre-oviposition period was longer in our sample of H. axyridis activated in June, probably due to the loss of a large proportion of nutrients (including proteins, Fig. 3) during the prolonged storage (eight months), although vitellogenin was still present (Fig. 5).
Fecundity
The prolonged cold storage of up to six months did not decrease the reproductive abilities of females. All subsamples except that for June (eight months of storage) started oviposition with similar rates of above 20 eggs per day per fertilized female. Thus, the use in biocontrol of field sampled H. axyridis is compatible with long storage until the required release time. However, fecundity of the stored females (except in the March sample) had substantially dropped already in the second month of post-winter reproduction, and the decrease in oviposition rate continued, resulting in the cessation of oviposition after about four months. Laboratory production thus should use only fresh females up to one month after activation, and then discard them. The progress of senescence was faster after longer storage, but in the June sample, even the initial fecundity was decreased and remained at that level for three months. Both the average and maximum number of eggs (daily and lifetime production) laid by females of H. axyridis are among the highest reported for ladybirds (Nedvěd and Honěk 2012) and thus justify the use of this species in biocontrol of aphids worldwide although the resulting invasion of this alien species has also deleterious consequences for biodiversity (Roy et al. 2012).
We confirmed and extended the results by Ruan et al. (2012) who found no adverse effect on reproductive capacity of H. axyridis after five months of storage at constant temperatures of 3 °C and 6 °C. Although the adults that experienced 90 day storage at 0 °C had the largest reproductive capacity, their survival substantially decreased after longer exposure to 0 °C. We did not intend to compare diverse long storage protocols or conditions (temperatures, humidity). We used periodic warming of the stored beetles for a few hours to enable drinking and repair chill injury as recommended by Nedvěd et al. (1998), Renault et al. (2004), Colinet et al. (2011) and Yocum et al. (2012). Although these conditions were artificially chosen, temperature and humidity also fluctuates in “natural” shelters.
Hatching rate
Females of the ladybird H. axyridis have a high capacity to maintain vital sperm during winter and use it for successful fertilization of eggs during several months after spring activation. Persistence of high levels of survival or vitality of sperm stored in the female spermatheca measured as egg hatching rate (percentage fertility) was longer than high levels of fecundity (number of eggs produced) of females. First, the initial hatching rate (during the first month after transfer from 6 to 20 °C) was similar (about 85 %) in all samples, from January to June. Thus survival of sperm during cold storage was high. Second, the percentage decreased only slightly in the second month after activation (to about 75 %) and gradually decreased up to the fifth or sixth month after activation (Fig. 2), more slowly than the fecundity decreased. Thus, sperm cells survived well also at ambient laboratory temperature (20 °C). The final decrease in hatching rate may represent either a decline in sperm viability over time at 20 °C or depletion of stored sperm with repeated ovipositions.
These results indicate the evolutionary importance of high incidence of pre-hibernation mating of ladybirds. They also explain that despite there being a lower survival rate of males during winter in comparison to females and therefore a small proportion of males observed in spring (29 %, unpublished observation) this does not cause a reduction in fertility and fitness of this generation.
Longer survival of sperm and slower decrease of hatching rate in comparison with fecundity indicates that old females of H. axyridis in spring do not use the strategy of laying trophic eggs as proposed by Perry and Roitberg (2005). Such unfertilized eggs would serve as food for freshly hatched first instar siblings to increase their survival before finding a proper food source. The lower proportion of fertilized eggs laid by old females would give them a comparative advantage and thus partially compensate for their lower number. However, we found that females used the opposite strategy—moderately old females still fertilized a relatively high percentage of eggs to produce a high number of offspring. Low fertilization rates of very old females then should be viewed as their inability to fertilize the small number of remaining eggs or inviability of the remaining sperm rather than an active strategy to produce trophic eggs. This last eventuality might be verified by mating the old females to young males. Lower hatching rate has been found in old females of several ladybird species (Propylea dissecta (Mulsant), Mishra and Omkar 2004, Coelophora saucia (Mulsant), Omkar et al. 2010) even if mated with young males.
Our study demonstrates persistence of high fecundity and fertility of females of the ladybird H. axyridis over a long storage at low temperature even when they were not mated after activation in suitable conditions and had to rely for the rest of their lives on the sperm supply stored through the winter period. Such high sperm survival questions the presumed need for multiple, frequent and long mating that has generally been observed in Coccinellidae. A single female of H. axyridis with such a high fertility may found a large new colony even without access to repetitive mating. Reproductive biology thus contributes to the striking invasive ability of this species.
References
Arnaud L, Haubruge E, Gage MJG (2001) Sperm size and number variation in the red flour beetle. Zool J Linn Soc 113:369–375
Berkvens N, Bonte J, Berkvens D, Tirry L, De Clercq P (2008) Influence of diet and photoperiod on development and reproduction of European populations of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). BioControl 53:211–221
Bind RB (2007) Reproductive behaviour of a generalist aphidophagous ladybird beetle Cheilomenes sexmaculata (Coleoptera: Coccinellidae). Int J Trop Insect Sci 27:78–84
Brown PMJ, Thomas CE, Lombaert E, Jeffries DL, Estoup A, Lawson Handley L-J (2011) The global spread of Harmonia axyridis (Coleoptera: Coccinellidae): distribution, dispersal and routes of invasion. BioControl 56:623–641
Ceryngier P, Kindlmann P, Havelka J, Dostalkova I, Brunnhofer V, Hodek I (1992) Effect of food, parasitization, photoperiod and temperature on gonads and sexual activity of males of Coccinella septempunctata (Coleoptera, Coccinellidae) in autumn. Acta Entomol Bohemoslov 89:97–106
Ceryngier P, Havelka J, Hodek I (2004) Mating and activity of gonads in pre-dormant and dormant ladybirds (Coleoptera: Coccinellidae). Invertebr Reprod Dev 45:127–135
Colinet H, Lalouette L, Renault D (2011) A model for the time-temperature-mortality relationship in the chill-susceptible beetle, Alphitobius diaperinus, exposed to fluctuating thermal regimes. J Thermal Biol 36:403–408
Danks HV (1987) Insect Dormancy: An Ecological Perspective, Biological Survey of Canada (Terrestrial Arthropods). Ottawa, Canada 439 pp
Gardner J, Hoffmann MP, Pitcher SA, Nyrop JP (2012) Recurrent warming to improve cold storage of trichogrammatids (Hymenoptera: Trichogrammatidae). Biocontrol Sci Technol 22:261–270
Gillott C (1988) Accessory sex glands in Arthropoda – Insecta. In: Adiyodi KG, Adiyodi RG (eds) Reproductive Biology of Invertebrates III. Accessory Sex Glands. John Wiley and Sons, UK, pp 319–373
Hanč Z, Nedvěd O (1999) Chill injury at alternating temperatures in Orchesella cincta (Collembola: Entomobryidae) and Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae). Eur J Entomol 96:165–168
Hodek I (2012) Dormancy. In: Hodek I, van Emden HF, Honek A (eds) Ecology and Behaviour of the Ladybird Beetles (Coccinellidae). Wiley-Blackwell, Oxford, UK, pp 275–342
Hodek I, Cerkasov J (1961) Experimental influencing of the imaginal diapauses in Coccinella septempunctata L. (Coccinellidae: Coleoptera). 7th contribution to the ecology of Coccinelidae. Acta Soc Zool Bohemoslov 25:70–90
Hodek I, Ceryngier P (2000) Sexual activity in Coccinellidae (Coleoptera): a review. Eur J Entomol 97:449–456
Iperti G, Bertand E (2001) Hibernation of Harmonia axyridis (Coleoptera: Coccinellidae) in South-Eastern France. Acta Soc Zool Bohemoslov 65:207–210
Laemmli UK (1970) Change of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Mishra G, Omkar (2004) Influence of parental age on reproductive performance of an aphidophagous ladybird, Propylea dissecta (Mulsant). J Appl Entomol 128:605–609
Nalepa CA, Kidd KA, Ahlstrom KR (1996) Biology of Harmonia axyridis (Coleoptera; Coccinellidae) in winter aggregations. Ann Entomol Soc Am 89:681–685
Nedvěd O, Honěk A (2012) Life history and development. In: Hodek I, van Emden HF, Honěk A (eds) Ecology and Behaviour of the Ladybird Beetles (Coccinellidae). Wiley-Blackwell, Oxford, UK, pp 54–109
Nedvěd O, Lavy D, Verhoef HA (1998) Modelling time temperature relationship in cold injury and effect of high temperature interruptions on survival in chill sensitive collembolan. Funct Ecol 12:816–824
Nedvědová T, Awad M, Ungerová D, Nedvěd O (2013) Characteristics of ladybird Harmonia axyridis during autumn migration. IOBC Bull (in press)
Obata S, Johki Y, Hidaka T (1986) Location of hibernation sites in the ladybird beetle, Harmonia axyridis. In: Conference: ecology of aphidophaga: proceedings of the 2nd symposium, Zvikovske, Podhradi, Czech Republic, pp 193–198
Obrycki JJ, Tauber MJ, Tauber CA, Gollands B (1983) Environmental control of the seasonal life cycle of Adalia bipunctata (Coleoptera: Coccinellidae). Environ Entomol 12:416–421
Okuda T, Chinzey Y (1988) Vitellogenesis in a lady beetle, Coccinella septempunctata in relation to the estivation diapause. J Insect Physiol 34:393–401
Omkar PA (2005a) Mating behavior of an aphidophagous ladybird beetle, Propylea dissecta (Mulsant). Insect Sci 12:37–44
Omkar MG (2005b) Mating in aphidophagous ladybirds: costs and benefits. J Appl Entomol 129:432–436
Omkar SS, Mishra G (2010) Parental age at mating affects reproductive attributes of the aphidophagous ladybird beetle, Coelophora saucia (Coleoptera: Coccinellidae). Eur J Entomol 107:341–347
Omkar MG, Srivastava S, Gupta AK, Singh SK (2005) Reproductive performance of four aphidophagous ladybirds on cowpea aphid, Aphis craccivora Koch. J Appl Entomol 129:217–220
Ornstein L (1969) Mechanism for very high sensitivity of Coomassie brilliant blue r250 (ICI) as a stain for protein. J Histochem Cytochem 17:189–192
Osawa N (2001) The effect of hibernation on the seasonal variations in adult body size and sex ratio of the polymorphic ladybird beetle Harmonia axyridis: the role of thermal melanism. Acta Soc Zool Bohemoslov 65:269–278
Perry JC, Roitberg BD (2005) Ladybird mothers mitigate offspring starvation risk by laying trophic eggs. Behav Ecol Sociobiol 58:578–586
Raak-van den Berg CL, Hemerik L, de Jong PW, van Lenteren JC (2012a) Mode of overwintering of invasive Harmonia axyridis in the Netherlands. BioControl 57:71–84
Raak-van den Berg CL, Stam JM, de Jong PW, Hemerik L, van Lenteren JC (2012b) Winter survival of Harmonia axyridis in the Netherlands. Biol Control 60:68–76
Renault D, Nedvěd O, Hervant F, Vernon P (2004) The importance of fluctuating thermal regimes for repairing chill injuries in the tropical beetle Alphitobius diaperinus (Coleoptera: Tenebrionidae) during exposure to low temperature. Physiol Entomol 29:139–145
Reznik SY, Vaghina NP (2011) Photoperiodic control of development and reproduction in Harmonia axyridis (Coleoptera: Coccinellidae). Eur J Entomol 108:385–390
Roy HE, Adriaens T, Isaac N, Kenis M, Onkelinx T, San Martin G, Brown PMJ, Hautier L, Poland R, Roy DB, Comont R, Eschen R, Frost R, Zindel R, van Vlaenderen J, Nedvěd O, Ravn HP, Grégoire JC, de Biseau J-C, Maes D (2012) Invasive alien predator causes rapid declines of native European ladybirds. Divers Distrib 18:717–725
Ruan CC, Du WM, Wang XM, Zhang JJ, Zang LS (2012) Effect of long-term cold storage on the fitness of pre-wintering Harmonia axyridis (Pallas). BioControl 57:95–102
Sakurai H, Hirano T, Takeda S (1987) Change in electrophoretic pattern of haemolymph protein in diapause regulation of the lady beetle, Coccinella septempunctata bruckii (Coleoptera: Coccinellidae). Appl Entomol Zool 22:286–291
Sakurai H, Kawai T, Takeda S (1992) Physiological changes related to diapause of the lady beetle, Harmonia axyridis (Coleoptera: Coccinellidae). Appl Entomol Zool 27:479–487
Sakurai H, Kumada Y, Takeda S (1993) Seasonal prevalence and hibernating diapause behaviour in the lady beetle, Harmonia axyridis. Res Bull Fac Agric Gifu Univ 58:51–55
Socha R, Sula J, Kodrik D, Gelbic I (1991) Hormonal control of vitellogenin synthesis In Pyrrhocoris apterus (L) (Heteroptera). J Insect Physiol 37:805–816
StatSoft Inc. (2011) STATISTICA (data analysis software system), version 10. http://www.statsoft.com
Tan CC (1946) Mosaic dominance in the inheritance of color patterns in the lady-bird beetle, Harmonia axyridis. Genetics 31:195–210
Wang S, Michaud JP, Tan XL, Zhang F, Guo XJ (2011) The aggregation behavior of Harmonia axyridis in its native range in Northeast China. BioControl 56:193–206
Yocum GD, Rinehart J, Kemp WP (2012) Duration and frequency of a high temperature pulse affect survival of emergence-ready Megachile rotundata (Hymenoptera: Megachilidae) during low-temperature incubation. J Econ Entomol 105:14–19
Acknowledgments
The study was undertaken within the framework of co-operation between Bulgarian Academy of Sciences and the Academy of Sciences of the Czech Republic. The study was supported by grant number QH82047 from the Ministry of Agriculture of the Czech Republic. We thank prof. Kodrik for his help with protein electrophoresis and Tom Fayle for language correction.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Patrick De Clercq.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Awad, M., Kalushkov, P., Nedvědová, T. et al. Fecundity and fertility of ladybird beetle Harmonia axyridis after prolonged cold storage. BioControl 58, 657–666 (2013). https://doi.org/10.1007/s10526-013-9512-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-013-9512-4