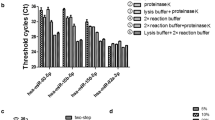
We performed a comparative quantitative analysis of LINE-1 mRNA levels in extracellular total plasma RNA in patients with colon cancer and practically healthy donors. Quantitative multiplex PCR with reverse transcription was used to assess the level of LINE-1 and 18S rRNA mRNA in extracellular total plasma RNA. The median of LINE-1 mRNA values in colon cancer patients (4.95) was significantly higher than in healthy donors (2.3) (p=0.037). It was shown for the first time that the level of LINE-1 mRNA in total RNA from blood plasma can be determined in the format of a liquid biopsy and serve as a new potential non-invasive marker of colon cancer.
Similar content being viewed by others
References
Adams JW, Kaufman RE, Kretschmer PJ, Harrison M, Nienhuis AW. A family of long reiterated DNA sequences, one copy of which is next to the human beta globin gene. Nucleic Acids Res. 1980;8(24):6113-6128. doi: https://doi.org/10.1093/nar/8.24.6113
Baba Y, Yagi T, Sawayama H, Hiyoshi Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Baba H. Long Interspersed Element-1 Methylation Level as a Prognostic Biomarker in Gastrointestinal Cancers. Digestion. 2018;97(1):26-30. doi: https://doi.org/10.1159/000484104
De Luca C, Guadagni F, Sinibaldi-Vallebona P, Sentinelli S, Gallucci M, Hoffmann A, Schumann GG, Spadafora C, Sciamanna I. Enhanced expression of LINE-1-encoded ORF2 protein in early stages of colon and prostate transformation. Oncotarget. 2016;7(4):4048-4061. doi: https://doi.org/10.18632/oncotarget.6767
Deniz Ö, Frost JM, Branco MR. Regulation of transposable elements by DNA modifications. Nat. Rev. Genet. 2019;20(7):417-431. doi: https://doi.org/10.1038/s41576-019-0106-6
Duggal NK, Emerman M. Evolutionary conflicts between viruses and restriction factors shape immunity. Nat. Rev. Immunol. 2012;12(10):687-695. doi: https://doi.org/10.1038/nri3295
Hancks DC, Kazazian HH Jr. Active human retrotransposons: variation and disease. Curr. Opin. Genet. Dev. 2012;22(3):191-203. doi: https://doi.org/10.1016/j.gde.2012.02.006
Kano H, Godoy I, Courtney C, Vetter MR, Gerton GL, Ostertag EM, Kazazian HH Jr. L1 retrotransposition occurs mainly in embryogenesis and creates somatic mosaicism. Genes Dev. 2009;23(11):1303-1312. doi: https://doi.org/10.1101/gad.1803909
Kulpa DA, Moran JV. Cis-preferential LINE-1 reverse transcriptase activity in ribonucleoprotein particles. Nat. Struct. Mol. Biol. 2006;13(7):655-660. doi: https://doi.org/10.1038/nsmb1107
Lander ES, Linton LM, Birren B. et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860-921. doi: https://doi.org/10.1038/35057062
Martin SL, Bushman FD. Nucleic acid chaperone activity of the ORF1 protein from the mouse LINE-1 retrotransposon. Mol. Cell Biol. 2001;21(2):467-75. doi: https://doi.org/10.1128/MCB.21.2.467-475.2001
Rodriguez-Martin B, Alvarez EG, Baez-Ortega A, Zamora J, Supek F, Demeulemeester J, Santamarina M, Ju YS, Temes J, Garcia-Souto D, Detering H, Li Y, Rodriguez-Castro J, Dueso-Barroso A, Bruzos AL, Dentro SC, Blanco MG, Contino G, Ardeljan D, Tojo M, Roberts ND, Zumalave S, Edwards PAW, Weischenfeldt J, Puiggròs M, Chong Z, Chen K, Lee EA, Wala JA, Raine K, Butler A, Waszak SM, Navarro FCP, Schumacher SE, Monlong J, Maura F, Bolli N, Bourque G, Gerstein M, Park PJ, Wedge DC, Beroukhim R, Torrents D, Korbel JO, Martincorena I, Fitzgerald RC, Van Loo P, Kazazian HH, Burns KH; PCAWG Structural Variation Working Group, Campbell PJ, Tubio JMC; PCAWG Consortium. Pan-cancer analysis of whole genomes identifies driver rearrangements promoted by LINE-1 retrotransposition. Nat. Genet. 2020;52(3):306-319. doi: https://doi.org/10.1038/s41588-019-0562-0
Solovyov A, Vabret N, Arora KS, Snyder A, Funt SA, Bajorin DF, Rosenberg JE, Bhardwaj N, Ting DT, Greenbaum BD. Global Cancer Transcriptome Quantifies Repeat Element Polarization between Immunotherapy Responsive and T Cell Suppressive Classes. Cell Rep. 2018;23(2):512-521. doi: https://doi.org/10.1016/j.celrep.2018.03.042
Ting DT, Lipson D, Paul S, Brannigan BW, Akhavanfard S, Coffman EJ, Contino G, Deshpande V, Iafrate AJ, Letovsky S, Rivera MN, Bardeesy N, Maheswaran S, Haber DA. Aberrant overexpression of satellite repeats in pancreatic and other epithelial cancers. Science. 2011;331(6017):593-596. doi: https://doi.org/10.1126/science.1200801
Walter M, Teissandier A, Pérez-Palacios R, Bourc’his D. An epigenetic switch ensures transposon repression upon dynamic loss of DNA methylation in embryonic stem cells. Elife. 2016;5:e11418. doi: https://doi.org/10.7554/eLife.11418
Wilhelm M, Wilhelm FX. Reverse transcription of retroviruses and LTR retrotransposons. Cell Mol. Life Sci. 2001;58(9):1246-1262. doi: https://doi.org/10.1007/PL00000937
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 173, No. 2, pp. 242-245, February, 2022
Rights and permissions
About this article
Cite this article
Filipenko, M.L., Boyarskikh, U.A., Leskov, L.S. et al. The Level of LINE-1 mRNA Is Increased in Extracellular Circulating Plasma RNA in Patients with Colorectal Cancer. Bull Exp Biol Med 173, 261–264 (2022). https://doi.org/10.1007/s10517-022-05530-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-022-05530-2