Abstract
Abalone farming produces nutrient-rich sludge, and the frequent cleaning and removal of sludge from abalone tanks is labour and capital-intensive. This study aimed to assess the effect of culture methods and tank cleaning frequency on abalone growth, water quality, and sludge characteristics. The study was conducted for 16 weeks. Four treatments were used, namely, abalone cocultured with sea cucumber cleaned once (AS1) and twice weekly (AS2); abalone monoculture cleaned once (A1) and cleaned twice weekly (A2). Abalone (initial avg weight = 40.9 g) were maintained under commercial stocking conditions in flow through systems at a salinity of 35 ppt and fed a commercial formulated diet (Abfeed™) and fresh seaweed (Ulva lacinulata and Gracillaria gracilis). The stocking densities were 200 abalone m−2 and 50 sea cucumbers m−2. The sea cucumbers (initial average weight = 12.5 g) fed on the abalone faeces and leftover feed, which settled at the abalone tank's bottom (sludge). There was a significant interaction between the type of culture and the frequency of cleaning on abalone weight (F (1,476) = 12.41, p < 0.001). Abalone in the A2 treatment group showed higher growth (p = 0.006) than those of the A1 treatment group, while abalone in AS1 had significantly higher growth (p < 0.001) than those in the AS2 treatment group. Also, abalone in AS1 showed higher growth (p = 0.026) than abalone in the A1 treatment. However, abalone in AS1 had growth similar (p = 0.53) to those in A2. The survival rate of the sea cucumbers was high, however, the sea cucumbers experienced a reduction in weight regardless of the treatment. This study showed that growth optimisation in abalone farming can be achieved by cleaning tanks twice instead of once weekly. However, when abalone are cocultured with sea cucumber, tanks need only be cleaned once a week without compromising abalone growth or water quality. The coculture of abalone and sea cucumber results in an additional aquacultured product, a significant reduction in labour and other associated costs of cleaning, without an additional cost of production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In aquaculture, wastes rich in nutrients such as nitrogen, carbon, phosphorous, and sulphur are derived from uneaten food, faeces, and other excreta of animals (Dauda et al. 2019). Solid wastes are sometimes referred to as sediment or sludge and settle at the bottom of tanks, ponds, or the benthos in open systems. The accumulation of sludge is often detrimental to the culture system and the animals therein. For example, excess sludge can reduce water quality by stimulating the proliferation of pathogenic bacteria (Rajkowski 2009). Infectious diseases are frequently caused by sludge build-up in the culture unit (Chen et al. 1997; Hossain et al. 2016; Khan 2018; Jasmin et al. 2020). In open systems, sludge impacts the receiving environment negatively and has led to criticism concerning aquaculture sustainability, including that of economically important abalone culture ( Piedrahita 2003; Cao et al. 2007; Bao et al. 2019; Ahmad et al. 2022).
Abalone is one of the most prized seafood delicacies in the world and is highly sought after, commanding a high monetary value (Cook 2016; Suleria et al. 2017). The depletion of abalone in the wild due to over-harvesting has led to abalone farming becoming established in many countries to supplement wild catches while contributing to food security (Hobday et al. 2000; Dichmont et al. 2000; Shepherd et al. 2001). South Africa is the third-largest producer of farmed abalone worldwide (HKTDC Research 2017). Haliotis midae is one of the abalone species endemic to South Africa and the only farmed species (DAFF 2018).
Farmed abalone are intensively fed with pellets and algae (Troell et al. 2009; Naidoo et al. 2006; Kirkendale et al. 2010). Abalone produce large amounts of feed waste (especially when fed on pellets) due to the animal's mode of feeding, scraping food particles before sending them to the mouth. In the process, food is broken into pieces (Mai et al. 1995). Waste food and faeces settle to form a layer of sludge at the base of abalone tanks. After some time, the sludge decomposes, affecting the tank's water quality, which is detrimental to abalone growth and health. This may result in stress, disease, and even death (Akinwole et al. 2016; Jasmin et al. 2020). Hence, cleaning abalone holding tanks frequently to remove sludge is a necessity. Traditional cleaning practices used in abalone farming include removing animals from the tank, draining rearing water, scrubbing the tank wall with a sponge and brush, and flushing out settled sludge with clean seawater. Traditional cleaning of abalone tanks is cumbersome, can lead to stress or cause physical harm to animals in culture, and a high percentage of production cost is spent on payment of labour for washing and tank cleaning (Lee et al. 2021). Traditional tank cleaning is especially laborious in the Eastern Cape of South Africa, located along the upper limit of the distribution of abalone, where water temperatures are usually high (average 21 °C) (Britz et al. 1997). These warmer temperatures lead to rapid bacterial proliferation in the sludge, resulting in tanks needing to be cleaned more frequently than in farms where the water is cooler.
Any innovation that reduces cleaning rates in abalone farming while maintaining animal growth and welfare without otherwise adding to the cost of production would be economically beneficial. Integrated multi-trophic aquaculture has been proposed as a possible ecological solution to reprocess and reduce nutrient release from aquaculture (Chopin et al. 2012; Zamora et al. 2018; Chary et al. 2020)."Integrated multi-trophic aquaculture (IMTA) is the farming, in proximity, of aquaculture species from different trophic levels, and with complementary ecosystem functions, in a way that allows one species' uneaten feed and wastes, nutrients, and by-products to be recaptured and converted into fertilizer, feed, and energy for the other crops, and to take advantage of synergistic interactions between species" (Chopin et al. 2012). The benefits of IMTA are increased efficiencies in terms of diversification, leading to increased profitability. A typical proposed potential IMTA candidate is the deposit-feeding sea cucumber. Sea cucumbers are valued food in Asia, commanding high prices (Prescott et al. 2017; Purcell et al. 2018). They are generally detritivores and are able to feed on aquaculture waste (Slater et al. 2009; Zamora and Jeffs 2012; Onomu et al. 2023). Sea cucumbers have been used as a coculture candidate in conjunction with abalone, shrimp, oysters, fish, scallops, mussels, and sea urchins (Kang et al. 2003; Slater and Carton 2007; Ren et al. 2012; Qi et al. 2013; Yokoyama 2015; Zhou et al. 2017; Neofitou et al. 2019; Grosso et al. 2021). Sea cucumbers possess bioremediation ability and can reduce waste's total organic matter, nitrogen, and carbon content via their feeding activity, thereby improving water quality (McTavish et al. 2012; Watanabe et al. 2012; Neofitou et al. 2019; Onomu et al. 2023). The cocultureof abalone and sea cucumber has reportedly led to improved growth in abalone (Kang et al. 2003; Bauer et al. 2019).
One factor that must be considered in IMTA is the compatibility of the species to be cocultured in order to prevent stress (Chopin et al. 2012). Stress or stress response refers to the reaction of an animal to the stressor. Stressors of aquatic animals include high stocking density, fluctuations in temperature, salinity, pH, and oxygen; inadequate food supply; and exposure to toxins (Cheng et al. 2004a, b; Lee et al. 2019). In vitro tests used as an indicator of stress or disease in cultured invertebrates, such as sea cucumber and abalone, include the haemocyte count, phenoloxidase measurement, superoxide production, phagocytosis assay, protein, glucose, and glycogen (Hooper et al. 2007).
Neostichopus grammatus is a species of sea cucumber endemic to South Africa and has been identified as a potential aquaculture species (Onomu et al. 2023). Neostichopus grammatus is not exploited in South Africa, i.e., it is not eaten, fished, or farmed. It is distributed from Cape Agulhas in Cape Town (34 "50'S/20"00'E) to Cape Vidal in Kwazulu Natal (28.3726°S, 32.4142°E) (Thandar 1987). The only study available on the culture of N. grammatus is that by Onomu et al. (2023) which reported the waste (faeces and leftover food) of abalone as an acceptable food to N. grammatus. The study also showed that N. grammatus can reduce the nitrogen, carbon and total organic matter content of waste via their feeding action. Thus N. grammatus was proposed as a potential IMTA candidate with abalone.
This study aims to assess the feasibility of the coculture of abalone (H. midae) and sea cucumber (N. grammatus) and apply principles of waste utilisation and integration in the same production system to address specific challenges encountered in abalone farming. The effect of tank cleaning frequency and method of culture (coculture/ monoculture system) on growth, water quality, sludge characteristics, and health are assessed.
Materials and Methods
Collection of experimental animals
Sea cucumbers (N. grammatus) were collected by Scuba divers west of Nahoon Reef (-32.98715°S, 27.96607°E), East London, Eastern Cape, South Africa. They were placed in 20 L buckets containing seawater and then transported to the study site (Wild Coast Abalone Farm) approximately two hours from the collection site. Hatchery-produced abalone, H. midae, were donated by Wild Coast Abalone Farm, Haga Haga, Eastern Cape.
Experimental design
The experiment ran for 16 weeks, with the experimental design consisting of four treatments with four tank replicates each. The treatments were: -
-
Abalone cocultured with sea cucumber with tanks cleaned once weekly (AS1);
-
Abalone cocultured with sea cucumber with tanks cleaned twice weekly (AS2);
-
Abalone monoculture with tanks cleaned once weekly (A1); and
-
Abalone monoculture with tanks cleaned twice weekly (A2).
Sixteen tanks were used for the study, which was constructed from Intermediate Bulk Container (IBC) referred to as "flow-bins"; the top of the flow-bin was cut off, and the remaining "tank" plumbed. The dimensions of the flow-bin tanks were 1 m x1m × 0.69 m (L x B x H). The tank had a water volume of 0.65 m3 and an inflow rate of two litres of seawater per minute, resulting in 0.31% of the total tank's volume being replaced per minute; the total tank volume was exchanged every 5 h. This exchange rate per basket of abalone was the same as that used in commercial abalone farms. Fourteen months old abalone was stocked in oyster mesh production baskets (L = 900 mm; W = 480 mm; H = 500 mm) according to standard abalone farm procedure (Fig. 1). Each tank contained an oyster mesh basket containing ca. 200 abalone with an average shell length of 59 mm and an average weight of 40.9 g or approx. 9.5 kg of abalone per basket resulting in a stocking density of 200 abalone m−2 and a biomass of 9.5 kg m−2 (abalone commercial stocking density). Each basket contained a rack that served as a substrate for the attachment of abalone.
A total of four hundred sea cucumbers with an average weight of approximately 12.5 g were stocked at the bottom of the abalone tanks at a density of 50 sea cucumbers (average weight = 628.59 ± 1.95 g) per tank. This resulted in a stocking density of 50 seacucumbers m−2 and a sea cucumber biomass of 628.59 ± 1.95 g m−2. The sea cucumber density was selected based on the feeding rate of the sea cucumber and the estimated waste and faeces produced by abalone as determined by Onomu et al. (2023). The sea cucumbers were provided with a dome-shaped PVC pipe in the tank base as a shelter.
Feeding
Abalone were fed as per standard farm procedure, which included being fed with a commercial abalone pellet- Abfeed™ 32S (32% protein; pellet size -10 mm x 10 mm × 1.2 mm; sinking feed), and fresh farm-produced seaweeds Ulva lacinulata, and Gracilaria gracilis. The animals were fed pellets daily (0.36% of body weight; feeding rate prescribed by the feed manufacturer) and seaweed (0.29% of body weight) thrice a week per standard farm procedure; this was adjusted as abalone increased in size. Of the three times feeding of seaweed, Ulva lacinulata was fed twice and Gracilaria gracilis once weekly. The amount of food given to the abalone daily was measured and used to calculate the average daily food provided, and if there was leftover food in the tanks, that particular tank was not fed. The sea cucumbers were not fed during the experimental period but had access to faeces and pellets that fell through the abalone basket onto the bottom of the tanks.
Cleaning and maintenance of tanks
Tanks were cleaned once or twice weekly, depending on the treatment. The cleaning routine involved moving the abalone baskets out of tanks and spraying with water from a hosepipe to unclog the mesh holes of debris, moving the washed basket into a recently filled tank with clean seawater, draining the original tank to about 20% to pick and transfer sea cucumbers underneath the tank into the new, cleaned tanks. Empty tanks were cleaned by scrubbing with a sponge and a brush, while baskets were sprayed only once weekly, irrespective of the cleaning frequency treatment. For tanks receiving twice-weekly cleaning, the second cleaning did not involve spraying the abalone basket but rather simply moving the basket into a washed tank containing clean seawater. In this manner, abalone and sea cucumbers from each treatment moved tanks in synchronization with each cleaning.
Survival
Survival of both abalone and sea cucumbers was monitored during the experiment. Survival was calculated as
Weight measurement
Wet weight (g) and shell length (mm) of 30 abalone (a representative number) were measured at the beginning of the study and every four weeks during the study using a weigh balance (Mettler PE 3600) and Caliper (Tork Craft). The sea cucumbers were starved for 48 h before the commencement of the study to evacuate guts content, according to Zamora and Jeffs (2012). Thereafter, the sea cucumbers were weighed. The sea cucumbers were placed on a sponge for a few seconds to expel excess coelomic fluid before being weighed. Growth was calculated as the mean tank change in weight.
Abalone feed conversion ratio (FCR)
The abalone baskets were monitored daily and fed only when no food was left in the basket (depicting that all feed was consumed as the pellet had high stability in water). The dry weight equivalent of the feed given to each (pellet and seaweed) was recorded and used for the FCR calculation. The FCR of abalone was estimated as
Amount of waste/ sludge generated
During each cleaning session (ca. every week and every three to four days—for once and twice cleaning, respectively), a mesh bag (180 µm) was tied to the outlet of the tanks. The faeces of abalone, either processed or unprocessed by the sea cucumber, and food that fell through the mesh of the abalone baskets were trapped by the mesh bag. The waste (faecal waste) was stored in a -20 °C deep freeze until it was ready to be processed for drying. The waste was dried using an oven to a constant weight at 70 °C, and the dry weight was measured using a weighing balance. Waste samples were not rinsed with fresh water to remove salt as some waste may be lost in the rinsing process, making the estimation of waste produced inaccurate. The salt in the waste was thought to be very minimal to influence the estimation accuracy of waste produced. To estimate the amount of waste generated per day, the dry weight of the sludge in each tank was divided by the cleaning session of the tank. That is, the dry weight of sludge derived from tanks cleaned once weekly was divided by seven, and the dry weight of those cleaned twice weekly were accumulated and divided by seven.
Proportion of feed turned waste per day (PFW)
The PFW was estimated as
Water quality
Water temperature was measured hourly with a hobo temperature logger UA-001–64 (Onset, USA). Dissolved oxygen was measured twice weekly with an OxyGuard Handy Polaris probe (OxyGuard International, Denmark); ammonia, nitrite, nitrate, and phosphorus were measured using a Palintest photometer 7100 (United Kingdom) every two weeks before routine tank cleaning.
Sludge assays
Three and six day old sludges collected from the base of abalone tanks at cleaning days (three replicates per treatment from separate tanks) were analysed for total organic matter, carbon, nitrogen, phosphorous, and sulphur content at weeks one, eight, and sixteen.
Total organic matter (TOM)
TOM was analysed using the combustion method (Byers et al. 1978). The samples were oven-dried at 60 °C for 48 h and weighed. Afterward, they were combusted in a furnace at 500 °C for 6 h. The samples were weighed, and TOM calculated as
Carbon and nitrogen
Carbon and nitrogen were determined by the Dumas combustion method using a C: N:S analyser (Carlo Erba Instruments NA Model 1500, USA). This was done by placing one mg of the sample in the tin container. The tin container was ignited at a high temperature of 1020 °C in oxygen, with the sample temperature rising to over 1500 °C on a second ignition of the tin container. An automatic analyzer equipped with a thermo-conductivity detector and a gas chromatographic separation column was used.
Phosphorous and sulphur
To analyse for phosphorus and sulphur, sludge samples were digested with nitric acid and perchloric acid using the open vessel wet digestion method, according to Zasoski and Burau (1977).
Health Indices
Collection of haemolymph and coelomic fluid
Abalone haemolymph (0.5 mL), was collected from the pedal sinus of abalone using 2 mL syringes and 26 G × 1/2″ needles, according to Macey and Coyne (2005). The haemolymph was placed into a microcentrifuge tube and placed on ice. The sea cucumbers were left on a sponge for a few seconds to expel sea water accumulated in the body, after which the sea cucumber was opened longitudinally and about 1 mL of coelomic fluid was collected according to the procedure of Zhang et al. (2021) and Luparello et al. (2019). The coelomic fluid was collected at the beginning of the study (control), and at the end of the study. The hemolymph and coelomic fluid samples were stored at -80 °C until required; and were used for glucose and protein analysis in abalone and sea cucumber, respectively. Coelomocytes of the sea cucumber coelomic fluid were separated by centrifuging at 3000 × g for 10 min at 4 °C. 400 µL coelomocytes were resuspended in 100 µL cold 0.85% saline and then sonicated at 22 kHz for 25 s, then centrifugation at 4000 g for 10 min, according to Chen et al. (2018). The resultant supernatant was used to determine superoxide dismutase.
Glucose
Abalone haemolymph was deproteinized by mixing 100ul of haemolymph with 100ul of 5% Trichloro acetic acid (TCA), which was left to react for 30 min. The mixture was then centrifuged at 13,000 × g. The supernatant was decanted for glucose analysis using the glucose oxidase procedure (GAGO-20, Sigma -Aldrich assay kit, USA). D-Glucose (Sigma-Aldrich, USA) was used as the standard.
Superoxide dismutase (SOD)
SOD was determined using a commercially available assay kit (Sigma-Aldrich: 19,160). The microplate was incubated at 37 °C for 20 min, and the colour was measured at 450 nm using a microplate reader (BioTek: SYNERGY Mx). One unit of SOD activity was defined as the amount of enzyme required for inhibiting superoxide-induced oxidation by 50%.
Protein
Protein was determined according to Bradford's method (Bradford 1976), with bovine serum albumin (Sigma-Aldrich: A7906) as the protein standard. A 5 µl volume of each sample and 250 µl of Bradford's reagent (Sigma-Aldrich: B6916) were added to a 96-well flat bottom plate (in triplicate). After incubating for 20 min, absorbance was measured at 595 nm using a microplate reader (BioTek: SYNERGY Mx).
Analysis of data
Sigma Plot Version 11 statistical software was used for all data analysis. At the commencement of the experiment, a one-way analysis of variance (ANOVA) was used to compare the mean tank weight of abalone to ensure that animal sizes and weights were statistically similar across the various treatments. T-test was used to compare the mean weight of sea cucumbers in co-culture. All data were tested for homogeneity (Levene’s test) and normality of variances (Shapiro–Wilk’s test). A two-factorial ANOVA was used to analyse the effect of both factors (culture method and tank cleaning frequency) on abalone weight, water quality parameters, sludge characteristics, and health parameters. Where a significant result was found (p < 0.05), Tukey's post hoc test was used for pairwise comparison.
Results
Survival
The survival rate of abalone in both monoculture and co-culture cleaned either once or twice was high over the duration of the study and showed no significant difference. The survival rate of abalone was 100 ± 0.00%, 95.5 ± 3.37%; 98.5 ± 0.58% and 99.5 ± 0.5% (mean ± SD) for AS1; AS2; A1, and A2, respectively. The survival rate of sea cucumbers was 92% for AS1 and AS2.
Water quality parameters
The culture method and tank cleaning frequency had no significant effect on the ammonia, dissolved oxygen, and pH levels. There were, however, differences in the levels of phosphorus, nitrate, and nitrite (Table 1). AS1 treatment had a significantly higher phosphorous level in water than AS2, A1 and A2. The frequency of cleaning influenced the nitrate level in the water such that tanks cleaned once (AS1 and A1) had a higher nitrate level compared to those cleaned twice (A2 and AS2). However, tanks cleaned twice (AS2 and A2) had a higher nitrite level in water than tanks cleaned once (AS1 and A1) (Table 1).
Abalone weight
When considering the target variable abalone weight, the two-way ANOVA revealed that the interaction term between the two factors, culture method and cleaning frequency, was significant (F (1,476) = 12.41, p < 0.001); i.e. differences among treatments cannot be attributed to only one or the other main factor. The frequency of cleaning alone had no effect on abalone weight (F = 0.136; p = 0.71); however, the culture method significantly influenced abalone weight (F = 6.93; p = 0.009). The results of Tukey's post hoc test show that abalone in the AS1 and A2 treatment groups had significantly higher weight (p < 0.001; p = 0.006 respectively) than abalone in the A1 treatment group (Fig. 2). Similarly, abalone in the AS1 treatment group had significantly higher weight (p = 0.026) than those in the AS2 treatment group. However, the weight of abalone in AS2 did not differ significantly (p = 0.53) from that of abalone in A2 treatment groups (Fig. 2).
Mean weight (g) of abalone in coculture and monoculture systems over 16 weeks of study. Where AS1 = abalone + sea cucumber + once weekly tank cleaning; AS2 = abalone + sea cucumber + twice weekly tank cleaning; A1 = abalone monoculture + once weekly tank cleaning, and A2 = abalone monoculture + twice weekly tank cleaning. Different letters above lines are significant (Tukey test, p < 0.05). Data are presented as mean ± SE (n = 30)
Abalone shell length
The interaction term between the two factors, culture method and cleaning frequency on abalone shell length, was significant (F (1,476) = 11.12; p < 0.001), meaning that differences among treatments cannot be attributed to only one or the main factor. The frequency of cleaning did not affect the shell length of the abalone (p = 0.43); however, the culture method significantly affected the shell length of the abalone (p = 0.004). The shell length of abalone in the AS1, AS2, and A2 treatment groups was similar to one another (p > 0.05) and significantly higher (p < 0.05) than that of A1 (Fig. 3).
Abalone’s mean shell length (mm) in coculture and monoculture systems over 16 weeks of study. Where AS1 = abalone + sea cucumber + once weekly tank cleaning; AS2 = abalone + sea cucumber + twice weekly tank cleaning; A1 = abalone monoculture + once weekly tank cleaning, and A2 = abalone monoculture + twice weekly tank cleaning. Different letters above lines are significant (Tukey , p < 0.05). Data are presented as mean ± SE (n = 30)
Sea cucumber growth
The weight of sea cucumbers in co-culture cleaned either once or twice was not significantly different at the end of the study (t = 0.242; p = 0.81). The sea cucumbers in both treatments showed a decrease in weight over the study period (Fig. 4).
Feed conversion rate (FCR) and proportion of feed turned waste per day (PFW)
The FCR, average daily food provided, average waste produced per day, and PFW of abalone in the various treatment were similar and were not influenced by the culture method or the tank cleaning frequency (Table 2). However, the interaction between the culture method and the frequency of tank cleaning was significant for PFW.
Sludge assays
Total organic matter (TOM)
At the end of the first week of the study, the culture method significantly influenced the sediment mean TOM concentration (F = 8.806; p = 0.018). However, there was no significant effect of cleaning frequency (F = 2.489; p = 0.153) on TOM, nor was there any interaction (F = 0.813; p = 0.394) between the two factors for TOM. However, at week 8, the culture method (F = 4.272; p = 0.073) and frequency of cleaning (F = 0.004; p = 0.953) had no significant effect on the mean TOM content of the sediment. Neither was a significant interaction term observed between factors (F = 0.913; p = 0.367). At week 16, the culture method had no significant effect (F = 0.586; p = 0.466) on the TOM of the sediment; however, the cleaning frequency significantly influenced the sediment's mean TOM. Tanks cleaned once had a higher sludge TOM content than those cleaned twice (F = 5.523; p = 0.047). The interaction term between the two factors' culture method and cleaning frequency on the sediments’ TOM was not significant (F (1,8) = 0.057; p = 0.817) (Fig. 5).
Mean TOM (%) of sludge from the coculture or monoculture systems over 16 weeks of study. Where AS1 = Abalone + sea cucumber + once weekly tank cleaning; AS2 = Abalone + sea cucumber + twice weekly tank cleaning; A1 = Abalone monoculture + once weekly tank cleaning and A2 = Abalone only + twice weekly tank cleaning). Data are presented as mean ± SE (n = 3). Different letters above each bar indicate a significant difference between treatments (p < 0.05)
Carbon
The carbon content of the sediments was similar (p > 0.05) among treatments in the first week of the study. Neither the culture method nor the frequency of tank cleaning used in the study showed a significant effect on the carbon content of the sediment in the first week (frequency of cleaning - F = 0.502; p = 0.499; culture method - F = 2.553; p = 0.149). The interaction term between the two factors, culture method and tank cleaning frequency on the carbon content, was insignificant (F (1,8) = 0.855; p = 0.382). Similarly, at week eight, the sediment carbon content showed no difference (p > 0.05). The culture method and the frequency of cleaning had no significant effect on the carbon content of the sediment at week eight (culture method - F = 0.159; p = 0.701; frequency of cleaning - F = 1.051; p = 0.336). Likewise, the interaction term between the two factors was insignificant (F (1,8) = 0.0501; p = 0.828).
However, at week 16, the frequency of cleaning had a significant effect on the sediment carbon content (F (1,8) = 14.845; p = 0.005) but not the culture method (F (1,8) = 0.013; p = 0.912). Tukey's post hoc test showed that tanks cleaned once had lower carbon content compared to those cleaned twice (Fig. 6).
Mean carbon (%) of sludge from the coculture or monoculture systems over 16 weeks of study. Where AS1 = Abalone + sea cucumber + once weekly tank cleaning; AS2 = Abalone + sea cucumber + twice weekly tank cleaning; A1 = Abalone monoculture + once weekly tank cleaning and A2 = Abalone monoculture + tanks cleaned twice weekly). Data are presented as mean ± SE (n = 3)
Nitrogen
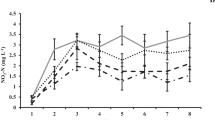
At the end of the first week of the study, the nitrogen content of sediment was not significantly affected by either the culture method (F = 0.404; p = 0.543) or the tank cleaning frequency (F = 1.850; p = 0.211). The interaction term between the two factors culture method and cleaning frequency was not significant (F (1,8) = 0.272; p = 0.616). Similarly, at the eighth week of the study, the nitrogen content of sediment was not significantly affected by either the culture method (F = 0.970; p = 0.354) or the tank cleaning frequency (F = 1.964; p = 0.199). The interaction term between the factors was not significant (F (1,8) = 1.125; p = 0.320). However, at week sixteen, the frequency of cleaning had a significant effect on the mean nitrogen content of the sediment but not the culture method (F = 0.082; p = 0.782). Tanks cleaned once had a lower sludge nitrogen content (F = 7.380; p = 0.026) than tanks cleaned twice at week 16. The interaction term between the factors, culture method and cleaning frequency on the nitrogen content of the sediment was not significant (F (1,8) = 0.283; p = 0.609) (Fig. 7).
Mean nitrogen (%) of sludge from the coculture or monoculture systems over 16 weeks of study. Where AS1 = Abalone + sea cucumber + once weekly tank cleaning; AS2 = Abalone + sea cucumber + twice weekly tank cleaning; A1 = Abalone monoculture + once weekly tank cleaning and A2 = Abalone monoculture + twice weekly tank cleaning). Data are presented as mean ± SE (n = 3). Different letters above each bar indicate a significant difference between treatments (Tukey, p < 0.05)
Phosphorus
After the first week, the culture method (F = 0.826, p = 0.390) and the frequency of cleaning (F = 5.163; p = 0.053) had no significant effect on the mean phosphorus content of the sediment. The interaction term between the two factors was not significant (F (1,8) = 0.242; p = 0.636). At week eight also, the culture method (F = 0.001; p = 0.981) and the frequency of cleaning (F = 0.001; p = 0.981) showed no significant effect on the phosphorus content of the sediment. The interaction effect between the two factors was not significant (F = 0.253; p = 0.628). Similarly, at week 16, the culture method (F = 0.834; p = 0.388) and the frequency of cleaning (F = 1.166; p = 0.312) had no effect on the mean phosphorus content of the sediment, and neither was the interaction term between the factors significant (F = 0.007; p = 0.936) (Fig. 8).
Mean phosphorus (%) of sludge from the coculture or monoculture systems over 16 weeks of study. Where AS1 = Abalone + sea cucumber + once weekly tank cleaning; AS2 = Abalone + sea cucumber + twice weekly tank cleaning; A1 = Abalone monoculture + once weekly tank cleaning and A2 = Abalone monoculture + twice weekly tank cleaning). Data are presented as mean ± SE (n = 3)
Sulphur
The culture method significantly affected (F = 5.384; p = 0.049) the mean concentration of sulphur in sediments in the first week of the study, unlike the frequency of cleaning, which was not significantly different (F = 1.253; p = 0.296). The mean sulphur content of sediment in co-culture was higher compared to those in the monoculture group, while no significant interaction was observed between the culture method and the tank cleaning frequency (F (1,8) = 0.913; p = 0.367). However, at weeks 8 and 16, the culture method (week 8 – F = 1.217; p = 0.302; week 16 – F = 1.112; p = 0.322) and the frequency of cleaning (week 8 – F = 2.565; p = 0.148 and week 16 – F = 4.085; p = 0.078) had no significant effect on the mean sulphur content of the sediment. There were also no significant interactions between these factors at week eight (F (1,8) = 0.923; p = 0.365) and week 16 (F (1,8) = 0.069; p = 0.799) (Fig. 9).
Mean sulphur (%) of sludge from the integrated multi-trophic aquaculture or monoculture systems over 16 weeks of study. Where AS1 = Abalone + sea cucumber + once weekly tank cleaning; AS2 = Abalone + sea cucumber + twice weekly tank cleaning; A1 = Abalone monoculture + once weekly tank cleaning and A2 = Abalone monoculture + twice weekly tank cleaning). Data are presented as mean ± SE (n = 3). Different letters above each bar indicate a significant difference between treatments (Tukey, p < 0.05)
Health indices
Abalone
The culture method and the frequency of cleaning had no significant effect on the haemolymph glucose, superoxide dismutase, and total protein (Table 3).
Sea cucumber
The coelomic protein, glucose, and coelomocyte superoxide dismutase values were similar between sea cucumbers in co-culture tanks cleaned once or twice and those from the control treatment (initial sea cucumber coelomocyte before the start of the experiment) (Table 4).
Discussion
Overall effect of tank cleaning frequency in relation to water quality and sludge parameter
Water quality parameters such as nitrite, nitrate, dissolved oxygen, and phosphorus are of great importance in aquaculture as they affect the growth, health, welfare, and well-being of aquatic animals (Boyd 2017; Xia et al. 2017; Liu et al. 2019; Motta et al. 2020).
The frequency of tank cleaning affected the nitrate and nitrite content of the rearing water such that tanks cleaned once weekly had higher nitrate and lower nitrite content when compared to tanks cleaned twice weekly, irrespective of the culture methods. The phosphorus content of the rearing water was influenced by the culture method and the tank cleaning frequency. Co-culture tanks cleaned once had a higher phosphorus content than co-culture tanks cleaned twice, and monoculture tanks cleaned either once or twice. The frequency of cleaning follows a natural phenomenon with some reactions that may either reduce or increase the concentrations and activeness of some chemical elements.
The frequency of cleaning used in this study significantly impacted the sludge characteristics in week 16 and not week eight or week 1 in both sections. Sludge characteristics not affected by the frequency of cleaning in weeks 1 and 8 could be due to the low water temperature experienced in these weeks (17.13 ± 0.06 °C; mean ± SE). However, as the water temperature began to show an increase from the eighth week (19.8 ± 0.06 °C; mean ± SE) difference in the sludge characteristics was observed. This result is similar to Prema et al. (2020), who stated that water temperature influences the decomposition rate as decomposition is faster at high temperatures than at low temperatures.
The sludge characteristics affected by the tank cleaning frequency were carbon and nitrogen. The sludge of tanks cleaned once weekly had lower levels of carbon and nitrogen than those cleaned twice weekly, irrespective of the presence/absence of sea cucumbers in tanks. This result could be because tanks cleaned once underwent more decomposition levels than those cleaned twice, resulting in more microorganisms to remineralize nutrients (Moriarty 1997; Liang and Blaser 2011; Boyd 2017). For example, (Blagodatsky et al. 2010) stated that soil decomposition rate is influenced by the size of the soil organic matter, the biomass of microorganism present, and the microbial activities. Similarly, (Jasmin et al. 2020) also stated that microorganisms play a role in the decomposition and mineralization of sludge and uses nutrient available in the sludge as nutrients and energy for growth.
Abalone growth
In aquaculture, the cleanliness of the culture environment is known to affect water quality, affecting the animal's welfare and growth (Lee et al. 2021). In the present study, abalone in monoculture tanks cleaned twice (A2) exhibited higher growth than those cleaned once (A1), possibly due to the frequency of cleaning (twice cleaning). Tanks cleaned twice may have a cleaner environment, composed of few microorganisms and lower microbial activity compared to those cleaned once. This is evidenced by the significantly increased water nitrate and nitrite contents in monoculture tanks cleaned only once weekly in the current study. Conversely, abalone in the co-culture tank cleaned once (AS1) exhibited significantly higher growth than those in the monoculture cleaned once (A1). This result is similar to previous findings on abalone and sea cucumber coculture, which report that abalone in coculture exhibits higher growth than those in monoculture (Kang et al. 2003; Bauer et al. 2019). The phenomena behind the presence of sea cucumber in an abalone tank cleaned once, resulting in higher growth of the abalone, has been linked not only to the consumption of waste but also the consumption of protozoans, bacteria, benthic diatoms, and macroalgae by the sea cucumber (Michio et al. 2003; ChávEz et al. 2011; Gao et al. 2011; Yokoyama 2015).
Abalone in the co-culture tank cleaned once (AS1) had similar growth to abalone in the monoculture tank cleaned twice (A2). It could be that the feeding activities of the sea cucumber in the co-culture tank cleaned once acted as a substitute for the cleaning effect achieved by cleaning tanks twice. While one might expect significantly improved abalone growth in the co-culture tank cleaned twice weekly (AS2) compared to those cleaned once weekly (AS1) because cleaning tanks twice means a healthier tank environment coupled with the presence of sea cucumbers since sea cucumbers play a role in improving growth while consuming waste. The reverse was the case.
This may indicate that the positive impact of the sea cucumbers on abalone in the co-culture tank cleaned twice (AS2) was not evident. This could be as a result of the increased cleaning frequency. The increased cleaning frequency and handling could cause stress to abalone, which impacted the growth but was too little to be detected by the health indicators used in this study (Morash and Alter 2016). Results of the current study revealed that an interaction exists between the culture methods and the frequency of tank cleaning applied. It can be argued that the frequency of tank cleaning affects the sea cucumber, which reduced feeding activity such that the bioremediation impact of the sea cucumber was not apparent. It could also be that frequent handling of abalone (twice instead of once) affected the growth performance of abalone in co-culture tanks cleaned twice (Morash and Alter 2016). This is corroborated by abalone growth in the co-culture tank cleaned twice (AS2) being similar to that of monoculture cleaned twice (A2). This raises questions such as:
1)What impact does twice cleaning have on sea cucumber in an abalone-sea cucumber co-culture system? And (2) what type of interaction/ reaction occurs in sea cucumber–abalone co-culture tanks cleaned twice weekly?
Sea cucumber growth
Several factors are known to affect the growth of cultured sea cucumbers, including spawning, stocking density, temperature, and the presence of sand substrate in tanks (An et al. 2007; Slater et al. 2009; Robinson et al. 2013; Bauer et al. 2019). Findings from the present study show that the sea cucumbers in co-culture tanks cleaned either once or twice lost weight in a way that did not significantly differ between treatments over the study period. The conditions in the tank, such as the tank bottom being bare, may have been unsuited to the sea cucumbers’ longer-term growth and could be the reason for the negative growth experienced by the sea cucumbers.
Nevertheless, the spawning event observed at the beginning of the experiment might have contributed to the weight loss experienced. The experimental period coincides with the period of natural spawning events in this species (September to November), during which the gonads of the sea cucumbers are reabsorbed, leading to weight loss (Foster and Hodgson 1995). The species of sea cucumber used in this study are naturally small and had attained sexual maturity as (Branch et al. 2016) reported that N. grammatus found in the wild does not attain more than 15 cm in size. Indices such as ingestion, faecal production and assimilation rate of sea cucumbers measured by Onomu et al. (2023) could have been beneficial in monitoring the welfare of the sea cucumbers. However, the present study was done on a larger scale compared to those of Onomu et al. (2023), which made measuring the indices not feasible. Also, since the sea cucumbers fed only on the waste (uneaten food and faecal waste) produced from the abalone, it was impossible to measure the amount of food ingested by the sea cucumber. Nevertheless, the bottom of the tanks was monitored during each cleaning event for faeces of sea cucumber, which served as evidence of waste consumption.
Health Indices
The superoxide dismutase, protein, and glucose content of abalone hemolymph and sea cucumber coelomocytes in the treatment groups were similar across all treatments. These results, compared to previous studies, indicate that the animals were not stressed. The values of SOD for abalone hemolymph recorded in this study are similar to Lange et al. (2014) and Vosloo et al. (2013a), who reported a SOD range of 0.09 – 0.13 U/mL for Haliotis laevigata and 0.08 – 2.18% mg/ protein for Haliotis midae, respectively. The result was, however, dissimilar from (Nam et al. 2020), who reported a range of 30 – 35 U/mL for the Pacific abalone. The results of the total protein of abalone recorded in this study were similar to that of Goosen et al. (2014), who reported a value of 8 – 10 mg/mL.
Glucose values of 23 – 25 mg/mL were recorded for Haliotis diversicolor (Cheng et al. 2004b) and 16- 20 mg/mL for Haliotis iris (Nollens et al. 2004). Similarly, Carefoot et al. (1993) showed 23- 25 mg/mL values for Haliotis kamtschatkana. However, values of 41.4 -99 mg/mL have been reported by (Vosloo et al. 2013a, b) for H. midae. The values of 24.69 -32.93 mg/mL recorded in this study are comparable and strongly indicate that the abalone in the current study were not stressed.
The glucose value of the sea cucumber coelomic fluid recorded in this study was similar to that of Chen et al. (2018), who recorded a glucose content of 9 mg/mL for A. japonicus. However, the SOD values recorded in this study were dissimilar to those reported by (Yan et al. 2014; Yu et al. 2020) (100 and 90 u/mL, respectively) for A. japonicus. Similarly, the values for the sea cucumber coelomic total protein reported in this study were lower than values obtained by (Hawa et al. 1999) (7.24 mg/mL, 2.05 mg/mL, and 1.80 mg/mL) for Bohadschia mamorata, Stichopus variegatus and Stichopus badiontus, respectively. The dis-similarities of the SOD and the total protein values compared to this study could be due to the different species of sea cucumber used. The values of the total protein and SOD recorded in this study show that the sea cucumber was not stressed, as the values were not different from those of the control recorded before the commencement of the experiment.
Indication of sea cucumber bioremediation
The culture methods (presence/ absence of sea cucumber) used in this study had no significant effect on the water quality parameters. This may be due to the high-water exchange rate (two litres per minute) used in this study. This exchange rate was used as it was the standard operating procedure of the farm where the study was carried out. This result is contrary to the findings of Kang et al. (2003), which stated that the co-culture of abalone (Haliotis discus) and sea cucumber (Stichopus japonicus) led to a reduction in inorganic nitrogen. However, it should be noted that Kang et al. (2003) used a static system which made it feasible to more clearly determine co-culture effect on water quality, unlike the present study with a high exchange rate.
The result shows that the culture methods (the presence/ absence of sea cucumber) had no significant effect on the sludge characteristics, regardless of string-like faeces observed in the tank as evidence that the sea cucumber utilized the sludge. The presence of sea cucumber showing little to no impact on the sludge could be due to the low water temperatures recorded in the study from week 1 to week 8. Similarly, sludge characteristics being unaffected by the presence of sea cucumber regardless of the increase in water temperature experienced after the eighth week of the study could be due to the increased abalone biomass, feeding rate and faecal production rate compared to the reduced sea cucumber biomass (half the initial weight), making the impact of the sea cucumber on the sludge unapparent. It could also be due to the short time frame the sea cucumber had (a maximum of 6 days) to feed on and impact the sediments before they were flushed out of the tank. This result is consistent with MacDonald et al. (2013), who reported similar carbon and nitrogen content of sediment impacted by Holothuria forskali and the control (without H. forskali) after five days of daily deposition of Dicentrarchus labrax waste. However, after an additional five days of non-deposition of D. labrax waste, the sediment impacted by H. forskali had a lower nitrogen content compared to the control (without H. forskali). In a similar experiment by MacDonald et al. (2013), the sea cucumber H. forskali was reared for eight weeks and fed with D. labrax waste. By the end of the experiment, lower carbon was reported for the sediment impacted by the sea cucumber than the control without sea cucumber.
Conclusion
This study aimed to assess the feasibility of co-culturing abalone (H. midae) and sea cucumber (Neostichopus grammatus) and the impact of tank cleaning frequency on abalone growth, water quality and sludge characteristics,
The results of this study confirm that growth optimisation in abalone farming (monoculture) can be achieved by cleaning tanks twice instead of once weekly. However, when abalone are cocultured with sea cucumbers, tanks need only be cleaned once a week without compromising abalone growth.The co-culture of the sea cucumber and abalone species led to improved abalone growth at the detriment of sea cucumber growth. Should the aim of co-culture be to obtain maximum growth of both species, including bioremediation, then the coculture of both species in this study might not be regarded as feasible. However, should the aim be to use sea cucumbers to enhance abalone growth and reduce labour costs involved in abalone tank cleaning (irrespective of the sea cucumber's growth), this form of coculture may be considered feasible.
Contrary to expectations, the frequency of tank cleaning proved to be the most crucial factor influencing the water quality and sludge characteristics. These findings could be beneficial not just to the abalone industry but to the aquaculture industry, where tank cleaning is a necessity. It is recommended that future research use smaller sea cucumbers at a low density which are provided a substrate (sand) to optimize their living conditions when in co-culture with abalone.
Data availability
The data that support the findings of this study are available on request from the corresponding author, A.J. Onomu.
References
Ahmad AL, Chin JY, Harun MHZM, Low SC (2022) Environmental impacts and imperative technologies towards sustainable treatment of aquaculture wastewater: A review. J Water Process Eng 46:102553
Akinwole AO, Dauda AB, Ololade AO (2016) Haematological response of Clarias gariepinus juveniles reared in treated wastewater after waste solids removal using alum or Moringa oleifera seed powder. Int J Acarology 6:1–8
An Z, Dong Y, Dong S (2007) Temperature effects on growth-ration relationships of juvenile sea cucumber Apostichopus japonicus (Selenka). Aquaculture 272:644–648. https://doi.org/10.1016/j.aquaculture.2007.08.038
Bao W, Zhu S, Jin G, Ye Z (2019) Generation, characterization, perniciousness, removal and reutilization of solids in aquaculture water: a review from the whole process perspective. Rev Aquacult 11:1342–1366. https://doi.org/10.1111/raq.12296
Bauer J, Vázquez-Vera L, Espinoza-Montes A et al (2019) Multi-trophic aquaculture of the green abalone Haliotis fulgens and the warty sea cucumber Apostichopus parvimensis enhances production. J Shellfish Res 38:455. https://doi.org/10.2983/035.038.0229
Blagodatsky S, Blagodatskaya E, Yuyukina T, Kuzyakov Y (2010) Model of apparent and real priming effects: Linking microbial activity with soil organic matter decomposition. Soil Biol Biochem 42:1275–1283. https://doi.org/10.1016/j.soilbio.2010.04.005
Boyd CE (2017) General relationship between water quality and aquaculture performance in ponds. In: Jene G (ed) Fish diseases. Academic Press, pp 147–166
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Branch G, Branch M, Griffiths C, Beckley L (2016) Two oceans: a guide to the marine life of southern Africa. Fourth Struik Nature, Cape Town
Britz PJ, Hecht T, Mangold S (1997) Effect of temperature on growth, feed consumption and nutritional indices of Haliotis midae fed a formulated diet. Aquaculture 152:191–203. https://doi.org/10.1016/S0044-8486(97)00002-1
Byers SC, Mills EL, Stewart PL (1978) A comparison of methods of determining organic carbon in marine sediments, with suggestions for a standard method. Hydrobiologia 58:43–47
Cao L, Wang W, Yang Y, Yang C, Yuan Z, Xiong S, Diana J (2007) Environmental impact of aquaculture and countermeasures to aquaculture pollution in China. Environ Sci Pollut Res-In 14:452–462
Chary K, Aubin J, Sadoul B et al (2020) Integrated multi-trophic aquaculture of red drum (Sciaenops ocellatus) and sea cucumber (Holothuria scabra): Assessing bioremediation and life-cycle impacts. Aquaculture 516:734621. https://doi.org/10.1016/j.aquaculture.2019.734621
ChávEz EA, Salgado-Rogel ML, Palleiro-Nayar J (2011) Stock Assessment of the warty sea cucumber fishery (Parastichopus parvimensis) of NW Baja California. California Cooperative Oceanic Fisheries Investigations Reports 52:136–147
Chen S, Coffin DE, Malone RF (1997) Sludge production and management for recirculating aquacultural systems. J World Aquaculture Soc 28(4):303–315
Chen J, Ren Y, Li Y, Xia B (2018) Regulation of growth, intestinal microbiota, non-specific immune response and disease resistance of sea cucumber Apostichopus japonicus (Selenka) in biofloc systems. Fish Shellfish Immunol 77:175–186. https://doi.org/10.1016/j.fsi.2018.03.053
Cheng W, Hsiao IS, Hsu CH, Chen JC (2004a) Change in water temperature on the immune response of Taiwan abalone Haliotis diversicolor supertexta and its susceptibility to Vibrio parahaemolyticus. Fish Shellfish Immunol 17:235–243
Cheng W, Li CH, Chen JC (2004b) Effect of dissolved oxygen on the immune response of Haliotis diversicolor supertexta and its susceptibility to Vibrio parahaemolyticus. Aquacultur 232:103e15
Chopin T, Cooper JA, Reid G et al (2012) Open-water integrated multi-trophic aquaculture: environmental biomitigation and economic diversification of fed aquaculture by extractive aquaculture. Rev Aquac 4:209–220
Cook PA (2016) Recent trends in worldwide abalone production. J Shellfish Res 35:581–583
DAFF (2018) Abalone Feasibility Study, Pretoria: DAFF
Dauda AB, Ajadi A, Tola-Fabunmi AS, Akinwole AO (2019) Waste production in aquaculture: Sources, components and managements in different culture systems. Aquaculture and Fisheries 4:81–88. https://doi.org/10.1016/j.aaf.2018.10.002
Dichmont CM, Buterworth DS, Cochrane KL (2000) Towards adaptive approaches to management of the South African Abalone Haliotis Midae Fishery. S Afr J Mar Sci 22:33–42. https://doi.org/10.2989/025776100784125753
Foster GG, Hodgson AN (1995) Annual reproductive cycles of three sympatric species of intertidal holothurians (Echinodermata) from the coast of the Eastern Cape Province of South Africa. Invertebr Reprod Dev 27:49–59. https://doi.org/10.1080/07924259.1995.9672433
Gao Q-F, Wang Y, Dong S et al (2011) Absorption of different food sources by sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea): Evidence from carbon stable isotope. Aquaculture 319:272–276. https://doi.org/10.1016/j.aquaculture.2011.06.051
Goosen NJ, de Wet LF, Görgens JF (2014) Rainbow trout silage oil as immunity enhancing feed ingredient in formulated diets for South African abalone Haliotis midae. Aquaculture 430:28–33. https://doi.org/10.1016/j.aquaculture.2014.03.040
Grosso L, Rakaj A, Fianchini A et al (2021) Integrated Multi-Trophic Aquaculture (IMTA) system combining the sea urchin Paracentrotus lividus, as primary species, and the sea cucumber Holothuria tubulosa as extractive species. Aquaculture 534:736268. https://doi.org/10.1016/j.aquaculture.2020.736268
Hawa I, Zulaikah M, Jamaludin M et al (1999) The potential of the coelomic fluid in sea cucumber as an antioxidant. Malays J Nutr 5:55–59
HKTDC Research (2017) Mainland Appetite for Abalone Sees Prices and Illicit Trade. [Online] Available at: http://economists-pick-research-miuat.hktdc.com/business-news/article/International-Market-News/Mainland-Appetite-for-Abalone-Sees-Prices-and-Illicit.TradeSoar/imn/en/1/1X000000/1X09ZQQ4.html
Hobday AJ, Tegner MJ, Haaker PL (2000) Over-exploitation of a broadcast spawning marine invertebrate: decline of the white abalone. Rev Fish Biol Fisheries 10:493–514
Hooper C, Day R, Slocombe R et al (2007) Stress and immune responses in abalone: Limitations in current knowledge and investigative methods based on other models. Fish Shellfish Immunol 22:363–379. https://doi.org/10.1016/j.fsi.2006.06.009
Hossain MA, Sarker AK, Amin MN, Hossain MM, Miah MS (2016) Development and performance evaluation of sludge remover for intensive aquaculture. Aquacult Eng 74:62–69
Jasmin MY, Syukri F, Kamarudin MS, Karim M (2020) Potential of bioremediation in treating aquaculture sludge: Review article. Aquaculture 519:734905. https://doi.org/10.1016/j.aquaculture.2019.734905
Kang KH, Kwon JY, Kim YM (2003) A beneficial coculture: charm abalone Haliotis discus hannai and sea cucumber Stichopus japonicus. Aquaculture 216:87–93. https://doi.org/10.1016/S0044-8486(02)00203-X
Khan MIR (2018) Shrimp Toilet: A novel way for disposal of organic waste in Aquaculture systems. Aquaculture International 52–54
Kirkendale L, Robertson-Andersson DV, Winberg PC (2010) Review on the use and production of algae and manufactured diets as feed for sea-based abalone aquaculture in Victoria. Shoalhaven Marine & Freshwater Centre 7. https://ro.uow.edu.au/smfc/7
Lange B, Currie K-L, Howarth GS, Stone DAJ (2014) Grape seed extract and dried macroalgae, Ulva lactuca Linnaeus, improve survival of greenlip abalone, Haliotis laevigata Donovan, at high water temperature. Aquaculture 433:348–360. https://doi.org/10.1016/j.aquaculture.2014.06.028
Lee J-W, Choi H, Hwang U-K et al (2019) Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Environ Toxicol Pharmacol 68:101–108. https://doi.org/10.1016/j.etap.2019.03.010
Lee JS, Pierce ML, Cook MA et al (2021) Effects of tank cleaning frequency and sea cucumber co-culture on larval sablefish growth and survival, water quality, and microbial communities. Aquaculture 545:737213
Liang C, Blaser TC (2011) Microbial production of recalcitrant organic matter in global soils: implications for productivity and climate policy. Nat Rev Microbiol 9:75
Liu B, Li X, Wang X, Huang B (2019) Effects of stocking density on stress response, innate immune parameters, and welfare of turbot (Scophthalmus maximus). Aquacult Int 27:1599–1612
Luparello C, Ragona D, Asaro DM et al (2019) Cytotoxic potential of the coelomic fluid extracted from the sea cucumber Holothuria tubulosa against triple-negative MDA-MB231 breast cancer cells. Biology 8(4):76
MacDonald CLE, Stead SM, Slater MJ (2013) Consumption and remediation of European Seabass (Dicentrarchus labrax) waste by the sea cucumber Holothuria forskali. Aquacult Int 21:1279–1290. https://doi.org/10.1007/s10499-013-9629-6
Macey BM, Coyne VE (2005) Improved growth rate and disease resistance in farmed Haliotis midae through probiotic treatment. Aquaculture 245:249–261. https://doi.org/10.1016/j.aquaculture.2004.11.031
Mai K, Mercer JP, Donlon J (1995) Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata L. and Haliotis discus hannai Ino. IV. Optimum dietary protein level for growth. Aquacultture 136:165–180
McTavish T, Stenton-Dozey J, Vopel K, Savage C (2012) Deposit-feeding sea cucumbers enhance mineralization and nutrient cycling in organically-enriched coastal sediments. PLoS ONE 7:e50031. https://doi.org/10.1371/journal.pone.0050031
Michio K, Kengo K, Yasunori K et al (2003) Effects of deposit feeder Stichopus japonicus on algal bloom and organic matter contents of bottom sediments of the enclosed sea. Mar Pollut Bull 47:118–125. https://doi.org/10.1016/S0025-326X(02)00411-3
Morash AJ, Alter K (2016) Effects of environmental and farm stress on abalone physiology: perspectives for abalone aquaculture in the face of global climate change. Rev Aquacult 8:342–368. https://doi.org/10.1111/raq.12097
Moriarty DJ (1997) The role of microorganisms in aquaculture ponds. Aquacultture 151:333–349
Motta JH, Glória LS, Polese MF et al (2020) Stocking density influences common carp larval development. Can restocking processes activate compensatory growth consequent to previous high stocking density? Span J Agric Res 18:e0608–e0608
Naidoo K, Maneveldt G, Ruck K, Bolton JJ (2006) A comparison of various seaweed-based diets and formulated feed on growth rate of abalone in a land-based aquaculture system. J Appl Phycol 18:437–443
Nam S-E, Haque MdN, Lee JS et al (2020) Prolonged exposure to hypoxia inhibits the growth of Pacific abalone by modulating innate immunity and oxidative status. Aquat Toxicol 227:105596. https://doi.org/10.1016/j.aquatox.2020.105596
Neofitou N, Lolas A, Ballios I et al (2019) Contribution of sea cucumber Holothuria tubulosa on organic load reduction from fish farming operation. Aquaculture 501:97–103. https://doi.org/10.1016/j.aquaculture.2018.10.071
Nollens HH, Keogh JA, Probert PK (2004) Haematological pathology of shell lesions in the New Zealand abalone, Haliotis iris (Mollusca: Gastropoda). Comp Clin Pathol 12:211–216. https://doi.org/10.1007/s00580-004-0503-9
Onomu A, Slater M, Vine N (2023) Feeding indicators and bioremediation ability of warty sea cucumber Neostichopus grammatus fed potential wastes from abalone Haliotis midae farming. Aquacult Environ Interact 15:45–57. https://doi.org/10.3354/aei00449
Piedrahita RH (2003) Reducing the potential environmental impact of tank aquaculture effluents through intensification and recirculation. Aquaculture 226(1–4):35–44
Prema D, Jenni B, Reena VJ, Vysakhan P, Prajitha K, Kripa V (2020) Water quality management in aquaculture. In: The Blue Bonanza: A Manual for on the Job Training Programme for VHSE Students on Advances in Fisheries & Aquaculture Techniques (Kochi: ICAR-Central Marine Fisheries Research Institute), pp 4–78. Available at: http://eprints.cmfri.org.in/14147/
Prescott J, Riwu J, Prasetyo AP, Stacey N (2017) The money side of livelihoods: Economics of an unregulated small-scale Indonesian sea cucumber fishery in the Timor Sea. Mar Policy 82:197–205
Purcell SW, Ngaluafe P, Lalavanua W, Ceccarelli DM (2018) Market price trends of Latin American and Caribbean sea cucumbers inform fisheries management. Regional Studies in Marine Science 17:127–132
Qi Z, Wang J, Mao Y et al (2013) Feasibility of Offshore Co-culture of Abalone, Haliotis discus hannai Ino, and Sea Cucumber, Apostichopus japonicus, in a Temperate Zone. J World Aquacult Soc 44:565–573. https://doi.org/10.1111/jwas.12056
Rajkowski KT (2009) Biofilms in fish processing. In: Fratamico PM, Annous BA, Gunther NW (eds) Biofilms in the Food and Beverage Industries. pp 499–516 https://doi.org/10.1533/9781845697167.4.499
Ren Y, Dong S, Qin C et al (2012) Ecological effects of co-culturing sea cucumber Apostichopus japonicus (Selenka) with scallop Chlamys farreri in earthen ponds. Chin J Ocean Limnol 30:71–79. https://doi.org/10.1007/s00343-012-1038-6
Robinson G, Slater MJ, Jones CLW, Stead SM (2013) Role of sand as substrate and dietary component for juvenile sea cucumber Holothuria scabra. Aquaculture 392–395:23–25. https://doi.org/10.1016/j.aquaculture.2013.01.036
Shepherd SA, Rodda KR, Vargas KM (2001) A chronicle of collapse in two abalone stocks with proposals for precautionary management. J Shellfish Res 20:843–856
Slater MJ, Carton AG (2007) Survivorship and growth of the sea cucumber Australostichopus (Stichopus) mollis (Hutton 1872) in polyculture trials with green-lipped mussel farms. Aquaculture 272:389–398. https://doi.org/10.1016/j.aquaculture.2007.07.230
Slater MJ, Jeffs AG, Carton AG (2009) The use of the waste from green-lipped mussels as a food source for juvenile sea cucumber, Australostichopus mollis. Aquaculture 292:219–224. https://doi.org/10.1016/j.aquaculture.2009.04.027
Suleria HAR, Masci PP, Gobe GC, Osborne SA (2017) Therapeutic potential of abalone and status of bioactive molecules: A comprehensive review. Crit Rev Food Sci Nutr 57(8):1742–1748
Thandar AS (1987) The southern African stichopodid holothurians, with notes on the changes in spicule composition with age in the endemic Neostichopus grammatus (HL Clark). African Zoology 22:278–286
Troell M, Joyce A, Chopin T et al (2009) Ecological engineering in aquaculture — Potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture 297:1–9. https://doi.org/10.1016/j.aquaculture.2009.09.010
Vosloo A, Laas A, Vosloo D (2013a) Differential responses of juvenile and adult South African abalone (Haliotis midae Linnaeus) to low and high oxygen levels. Comp Biochem Physiol a: Mol Integr Physiol 164:192–199. https://doi.org/10.1016/j.cbpa.2012.09.002
Vosloo D, van Rensburg L, Vosloo A (2013b) Oxidative stress in abalone: The role of temperature, oxygen and l-proline supplementation. Aquaculture 416–417:265–271. https://doi.org/10.1016/j.aquaculture.2013.09.031
Watanabe S, Kodama M, Zarate JM, Lebata-Ramos MJ, Nievales MF (2012) Ability of sandfish (Holothuria scabra) to utilise organic matter in black tiger shrimp ponds. In: Proceedings Asia− Pacific tropical sea cucumber aquaculture. Australian Centre for International Agricultural Research, Canberra, pp 113–20
Xia B, Ren YC, Wang JY et al (2017) Effects of feeding frequency and density on growth, energy budget and physiological performance of sea cucumber Apostichopus japonicus (Selenka). Aquacultture 466:26–32. https://doi.org/10.1016/j.aquaculture.2016.09.039
Yan F, Tian X, Dong S et al (2014) Growth performance, immune response, and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus fed a supplementary diet of the potential probiotic Paracoccus marcusii DB11. Aquaculture 420–421:105–111. https://doi.org/10.1016/j.aquaculture.2013.10.045
Yokoyama H (2015) Suspended culture of the sea cucumber Apostichopus japonicus below a Pacific oyster raft – potential for integrated multitrophic aquaculture. Aquac Res 46:825–832. https://doi.org/10.1111/are.12234
Yu Z, Robinson S, MacDonald B et al (2020) Effect of diets on the feeding behavior and physiological properties of suspension-feeding sea cucumber Cucumaria frondosa. J Ocean Limnol 38:883–893. https://doi.org/10.1007/s00343-019-9190-x
Zamora LN, Jeffs AG (2012) The ability of the deposit-feeding sea cucumber Australostichopus mollis to use natural variation in the biodeposits beneath mussel farms. Aquaculture 326–329:116–122. https://doi.org/10.1016/j.aquaculture.2011.11.015
Zamora LN, Yuan X, Carton AG, Slater MJ (2018) Role of deposit-feeding sea cucumbers in integrated multitrophic aquaculture: progress, problems, potential and future challenges. Rev Aquacult 10:57–74. https://doi.org/10.1111/raq.12147
Zasoski RJ, Burau RG (1977) A rapid nitric-perchloric acid digestion method for multi-element tissue analysis. Commun Soil Sci Plant Anal 8:425–436
Zhang C, Yu Z, Xue Z, Li H, Zhu J, Wang L, Song L (2021) The temporal dynamics of bacteria in the coelomic fluid of sea cucumber Apostichopus japonicus after evisceration. Invertebrate Survival J, 46–55
Zhou S, Ren Y, Pearce CM et al (2017) Ecological effects of co-culturing the sea cucumber Apostichopus japonicus with the Chinese white shrimp Fenneropenaeus chinensis in an earthen pond. Chin J Ocean Limnol 35:122–131. https://doi.org/10.1007/s00343-016-5184-0
Acknowledgements
The authors would like to thank the management and staff of Wild Coast Abalone Farm (Pty) Ltd, especially, Sanet Petschel, Noel Marshal, and Johnathan Philander, for providing experimental animals, infrastructure, and facilities. Immense appreciation also goes to Daphne Taylor, for her support during the project. The authors thank the reviewers of this paper for their thorough and constructive peer reviews.
Funding
Open access funding provided by University of Fort Hare. This project is part of Aqua Vitae and was partly funded by European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 818173. The University of Fort Hare’s Govan Mbeki Research and Development Center (GMRDC) and the National Research Foundation’s (NRF) Research Technology Fund (Grant: 135443) are acknowledged for the provision of a bursary to A.J.O. and project funding.
Author information
Authors and Affiliations
Contributions
This paper formed part of the research contributing to the PhD thesis of A.J.O. All authors contributed to the study conception and design. Project funding and bursaries were sourced by M.J.S. and N.G.V. Material preparation, data collection and analysis were performed by A.J.O. The first draft of the manuscript was written by A.J.O and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Onomu, A.J., Slater, M.J. & Vine, N.G. Coculture of abalone (Haliotis midae) and sea cucumber (Neostichopus grammatus) to reduce tank cleaning frequency in abalone farming. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01508-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01508-1