Abstract
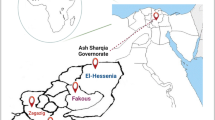
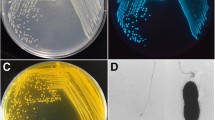
Tenualosa ilisha popularly known as ‘Hilsa’ is one of the most commercially important euryhaline food fish species in South Asian countries. Co-infection of Fusarium incarnatum FHHS2 and Acinetobacter bohemicus FHHK1 was reported for the first time in captive-reared pre-adult T. ilisha (average length 22.30 ± 0.92 cm and weight 93.48 ± 28.06 g) that had manifested skin lesions in freshwater pond during November–December 2022. The infected fish showed lethargy, abnormal swimming behaviour, blackening of gills, red streaks on the fins, erosions of the skin, and sloughing of the scales resulting in 40% mortality of the stock. Microscopic observation revealed the presence of aerial slightly curved conidia, septate macroconidia, and randomly spread microconidia. The fungal species was confirmed as F. incarnatum FHHS2 (NCBI GenBank accession number OR030402) by ITS sequencing. It clustered with known pathogenic F. incarnatum (NCBI Accession no. AY633745) counterpart associated with black gill disease of Penaeus monodon Fabricius (99.46% homology) in phylogenetic tree. We have also isolated, identified, and characterised A. bohemicus FHHK1 from the infected fish which clustered with A. bohemicus reported in the lakes of Indian subcontinent (100.00% homology). No parasite was found in different organs of the moribund hilsa shad. Significant histopathological alterations were observed in the skin, gill, and kidney of infected hilsa. Our study records the first report of F. incarnatum FHHS2 and A. bohemicus FHHK1borne infection in hilsa during its domestication in freshwater pond.








Similar content being viewed by others
Data availability
Available upon request from the corresponding author of this article.
References
Abd El-Ghany NA, El-Khatib NR, Salama SSA (2014) Causes of mortality in discus fish (Symphysodon) and trials for treatment. Egypt J Aquac 4:1–12
Akther M, Alam A, D’silva J, Bhuiyan AI, Bristow GA, Berland B (2004) Goezia bangladeshi n. sp. (Nematoda, Anisakidae) from an anadromous fish Tenualosa ilisha (Clupeidae). J Helminthol 78:105–113
Alderman DJ, Polglase JL (1985). Fusarium tabacinum (Beyma) Gams, as a gill parasite in the crayfish, Austropotamobius pallipes Lereboullet. J Fish Dis 8(2):249–252
APHA (2005) Standard methods for examination of water and wastewater, 21st edn. American Public Health Association, American Water Works Association, Water Pollution Control Federation, New York
Austin B, Austin DA (2016) In: Bacterial fish pathogens: disease of farmed and wild fish, 6th edn. Springer International Publishing, Switzerland
Balmas V, Migheli Q, Scherm B, Garau P, O’Donnell K, Ceccherelli G et al (2010) Multilocus phylogenetics show high levels of endemic fusaria inhabiting Sardinian soils (Tyrrhenian Islands). Mycol 102(4):803–812
Banerjee SM (1967) Water quality and soil condition of fishponds in states of India in relation to fish production. Indian J Fish 14:115–144
Behera B, Paria P, Das A, Bhowmick S, Sahoo A, Das BK (2017) Molecular characterization and pathogenicity of a virulent Acinetobacter baumannii associated with mortality of farmed Indian Major Carp Labeo rohita (Hamilton 1822). Aquac 471:157–162
Bhuiyan AI, D’silva J, Bristow GA (2007) The metazoan ectoparasite community structure on the gills of the hilsa shad Tenualosa ilisha (Clupeidae) in Bangladesh. Dhaka Univ J Biol Sci 16(1):1–10
Bhuiyan AI, D’silva J, Bristow GA (2009) Parasites of hilsa shad, Tenualosa ilisha in Bangladesh. Bangladesh J Zool 37(2):221–230
Bhuiyan AI, Momen M (2012) Studies on the protozoan parasites of hilsa shad, Tenualosa ilisha in Bangladesh. Bangladesh J Zool 40(1):33–41
Bisht D, Bisht GS, Khulbe RD (2000) Fusarium–a new threat to fish population in reservoirs of Kumaun, India. Curr Sci 78:1241–1245
Boyd CE (1979) Determination of total ammonia nitrogen and chemical oxygen demand in fish culture systems. Trans Am Fish Soc 108(3):314–319
Boyd CE (1982) Water quality management for pond fish culture. Elsevier Sci. Publ. Co., Amsterdam-Oxford-New York-Tokyo, pp 318–320
Boyd CE, Tucker CS (1998) Pond aquaculture and water quality management. Kluwer Academic Pub, London, pp 44–48
Bruemmer TM, Lewbart G (1996) Fusarium solani infection in a scrawled file fish (Aluterus scriplus) IAAAM Archieve. https://www.vin.com/doc/?id=3981767. Accessed 23 January 2023
Cabanes FJ, Alonso JM, Castellá G, Alegre F, Domingo M, Pont S (1997) Cutaneous hyalohyphomycosis caused by Fusarium solani in a loggerhead sea turtle (Caretta caretta L.). J Clin Microbiol 35(12):3343–3345
Cao S, Geng Y, Yu Z, Deng L, Gan W, Wang K et al (2018) Acinetobacter iwoffii, an emerging pathogen for fish in Schizothorax genus in China. Transbound Emerg Dis 65(6):1816–1822
Chehri K (2015) Morphological and phylogenetic analysis of Fusarium solani species complex in Malaysia. Microb Ecol 69:457–471
Collins MD, Samelis J, Metaxopoulos J, Wallbanks S (1993) Taxonomic studies on some leuconostoc-like organisms from fermented sausages: description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J Appl Bacteriol 75(6):595–603
Crow GL, Brock JA, Kaiser S (1995) Fusarium solani fungal infection of the lateral line canal system in captive scalloped hammerhead sharks (Sphyma lewini) in Hawaii. J wildl Dis 31(4):562–565
Cutuli MT, Gibello A, Rodriguez-Bertos A, Blanco MM, Villarroel M, Giraldo A et al (2015) Skin and subcutaneous mycoses in tilapia (Oreochromis niloticus) caused by Fusarium oxysporum in coinfection with Aeromonas hydrophila. Med Mycol Case Rep 9:7–11
Desoubeaux G, Debourgogne A, Wiederhold NP, Zaffino M, Sutton D, Burns RE et al (2018) Multi-locus sequence typing provides epidemiological insights for diseased sharks infected with fungi belonging to the Fusarium solani species complex. Med Mycol 56:591–601
D'Silva J, Bhuiyan AI, Bristow GA (2012) Distribution of helminth parasites in size groups and organs of hilsa shad, Tenualosa ilisha. Dhaka Univ J Biol Sci 21(1):55–65
Duc PM, Thy DTM, Hatai K, Muraosa Y (2015) Infection of striped catfish (Pangasianodon hypophthalmus) in Viet Nam caused by the fungus Fusarium incarnatum-equiseti. Bull Eur Ass Fish Pathol 35(6):208–216
Fernando N, Hui SW, Tsang CC et al. (2015) Fatal Fusarium solani species complex infections in elasmobranchs: the first case report for black spotted stingray (Taeniura melanopsila) and a literature review. Mycoses 58(7):422–431
Foroutan M, Dalimi A, Ghaffari AD (2020) The first report of Faustula keksooni from Tenualosa ilisha in Karun River, southwest of Iran. Infect Disord Drug Targets 20(6):898–901
Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ (2008) Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol 74(8):2461–2470
Garner MM (2013) A retrospective study of disease in elasmobranchs. Vet Pathol 50(3):377–389
Geiser DM (2004) FUSARIUM-ID v. 1.0: a DNA sequence database for identifying Fusarium. Eur J Plant Pathol 110:473–479
Hassan O, Hassan A, Abd El Ghany N, El-baky A, Hanna M, Abd El Aziz M (2020) A contribution on the pathogenicity of Fusarium oxysporum isolated from cultured Nile tilapia (Oreochromis niloticus) with trials for the treatment. Egypt J Aquat Biol Fish 24(5):197–215
Hatai K, Kubota SS, Kida N, Udagawa SI (1986). Fusarium oxysporum in red sea bream (Pagrus sp.). J Wildl Dis 22(4):570–571
Hatai K (2012) Diseases of fish and shellfish caused by marine fungi. In: Biology of marine fungi, progress in molecular and subcellular biology. Springer-Verlag, Berlin, Heidelberg, pp 15–52
Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST (1994) In: Bergey’s manual of determinative bacteriology. Williams and Wilkins Press, Baltimore, USA
Hoque F, Adhikari S, Mandal RN, Hussan A, Chattopadhyay D, Paul BN et al (2022) A retrospective study on the incidence of fish diseases and use of therapeutants in aquaculture farms of Moyna, the ‘fisheries hub’ of west bengal, india. Explor Anim Med Res 12(2):195–204
Jhingran VG (1975) Fish and fisheries of India. Hindustan Publishing Corporation (India), Delhi
Kathuria S, Singh PK, Sharma C, Prakash A, Masih A, Kumar A et al (2015) Multidrug-resistant Candida auris misidentified as Candida haemulonii: characterization by matrix-assisted laser desorption ionization–time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CLSI broth microdilution, and Etest method. J Clin Microbiol 53(6):1823–1830
Khoa LV, Hatai K, Aoki T (2004) Fusarium incarnatum isolated from black tiger shrimp, Penaeus monodon Fabricius, with black gill disease cultured in Vietnam. J Fish Dis 27(9):507–515
Khoa LV, Hatai K (2005) First case of Fusarium oxysporum infection in cultured Kuruma prawn Penaeus japonicus in Japan. Fish Pathol 40:195–196
Kozińska A, Paździor E, Pękala A, Niemczuk W (2014) Acinetobacter johnsonii and Acinetobacter lwoffii- the emerging fish pathogens. Bull Vet Inst Pulawy 58:193–199
Kulatunga DCM, Dananjaya SHS, Park BK, Kim CH, Lee J, De Zoysa M (2016) First report of Fusarium oxysporum species com- plex infection in zebrafish culturing system. J Fish Dis 40:485–494
Lechevalier M (1980) The chemotaxonomy of actinomycetes. Actinomycete Taxonomy, pp 227–291
Leslie JF, Summerell BA (2008) The Fusarium laboratory manual. John Wiley & Sons
Li J, Cao J, Wang X, Liu N, Wang W, Luo Y (2017) Acinetobacter pittii, an emerging new multi-drug resistant fish pathogen isolated from diseased blunt snout bream (Megalobrama amblycephala Yih) in China. Appl Microbiol Biotechnol 101(16):6459–6471
Lydia S, St. Leger J, Bossart G, Townsend FI Jr, Hicks C, Rinaldi M (2010) A novel case of Fusarium oxysporum infection in an atlantic bottlenose dolphin (Tursiops truncatus). J Zoo Wildl Med 41:287–290
MacFaddin JF (1980) In: Biochemical tests for identification of medical bacteria, 2nd edn. Waverly Press, Inc, USA ISBN 0-68305315-9
Mallik SK, Shahi N, Joshi N, Pant K, Kala K, Chandra S et al (2020) The emergence of zoonotic Fusarium oxysporum infection in captive-reared fingerlings of golden mahseer, Tor putitora (Hamilton, 1822) from the central Himalayan region of India. Transbound Emerg Dis 67(2):555–563
Marancik D, Collins J, Afema J, Lawrence C (2020) Exploring the advantages and limitations of sampling methods commonly used in research facilities for zebrafish health inspections. Lab Anim 54(4):373–385
Muhvich AG, Reimschuessel R, Lipsky MM, Bennett RO (1989) Fusarium solani isolated from newborn bonnethead sharks, Sphyrna tiburo (L.). J Fish Dis 12(1):57–62
Nirenberg HI, O’Donnell K (1998) New Fusarium species and combinations within the Gibberella fujikuroi species complex. Mycol 90:434–458
OIE (2006) In: Manual of diagnostic tests for aquatic animals, 5th edn. World Organisation for Animal Health, Paris
OIE (2013) In: Aquatic animal health code (16th edn) World Organisation for Animal Health, Paris, France, 284
OIE (2021) Manual of diagnostic tests for aquatic animals. The World Organisation for Animal Health, Paris, France https://www.oie.int/en/standard-setting/aquatic-manual/access-online/
Pękala-Safińska A, Jedziniak P, Kycko A, Ciepliński M, Paździor E, Panasiuk Ł et al (2020) Could mycotoxigenic Fusarium sp. play a role in ulcerative dermal necrosis (UDN) of brown trout (Salmo trutta morpha trutta)? Mycotoxin Res 36(3):311–318
Rahman MJ, Wahab MA, Nahiduzzaman M, Haque ABMM, Cohen P (2020) Hilsa fishery management in Bangladesh. IOP Conference Series: Earth and Environmental Science (414(1): 012018. IOP Publishing.
Rauta PR, Kumar K, Sahoo PK (2011) Emerging new multi-drug resistant bacterial pathogen, Acinetobacter baumannii associated with snakehead Channa striatus eye infection. Curr Sci 101(4):548–553
Roberts RJ (2012) Fish pathology, 4th edn. Wiley-Blackwell, UK, p 581
Roy A, Manna RK, Sharma AP (2016) Socio-economic and livelihood analyses of Hilsa (Tenulosa ilisha) fishers of lower stretch of Ganga River, India. Indian J Fish 63:83–88
Sahoo AK, Wahab MA, Phillips M, Rahman A, Padiyar A, Puvanendran V et al (2018) Breeding and culture status of Hilsa (Tenualosa ilisha, Ham. 1822) in South Asia: a review. Rev Aquac 10(1):96–110
Salter CE, O’Donnell K, Sutton DA, Marancik DP, Knowles S, Clauss TM (2012a) Dermatitis and systemic mycosis in lined seahorses Hippocampus erectus associated with a marine-adapted Fusarium solani species complex pathogen. Dis Aquat Organ 101:23–31
Salter CE, O’Donnell K, Sutton DA, Marancik DP, Knowles S, Clauss TM et al (2012b) Dermatitis and systemic mycosis in lined seahorses Hippocampus erectus associated with a marineadapted Fusarium solani species complex pathogen. Dis Aquat Organ 101:23–31
Sautour M, Edel-Hermann V, Steinberg C, Sixt N, Laurent J, Dalle F et al (2012) Fusarium species recovered from the water distribution system of a French university hospital. Int J Hyg Environ Health 215:286–292
Saylor KR, Miller LD, Vandersea WM, Bevelhimer SM, Schofield JP, Bennett AW (2010) Epizootic ulcerative syndrome caused by Aphanomyces invadans in captive bullseye snakehead Channa marulius collected from South Florida, USA. Dis Aquat Organ 88:169–175
Slepecky RA, Hemphill HE (2006) The genus Bacillus-nonmedical. Chapter 1.2.16. Prokaryotes 4:530–562
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027
Thompson JD, Gibson TJ, Higgins DG (2003) Multiple sequence alignment using Clustal W and Clustal X. Curr Protoc Bioinformatics 1:2–3
Uğur A, Ceylan Ö, Aslım B (2012) Characterization of Pseudomonas spp. from seawater of the southwest coast of Turkey. J Biol Environ Sci 6(16):15–23
Violetta GC, Dalton LM, Crawley R (1989) A case history of Fusarium sp. in a captive population of bonnethead sharks, Sphyrna tiburo. In IAAAM Conf. Proceed 20:64
Wahab MA, Beveridge MCM, Phillips MJ (2019) Hilsa: status of fishery and potential for aquaculture. 16. World Fish. Proceedings, Penang, Malaysia, p 196
Weatherburn MW (1967) Phenol-hypochlorite reaction for determination of ammonia. Anal Chern 39:971–974
Xia L, Xiong D, Gu Z, Xu Z, Chen C, Xie J et al (2008) Recovery of Acinetobacter baumannii from diseased channel catfish (Ictalurus punctatus) in China. Aquac 284(1–4):285–288
Yanong RP (2003) Fungal diseases of fish. Vet Clin North Am Exot Anim Pract 6(2):377–400
Funding
The research work was funded by ICAR-National Agricultural Science Fund (NASF) Grant no. NASF/ABA(SM)-8026/2020-21.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. F.H.: conceptualization, methodology, data curation, formal analysis, validation, writing original draft preparation, review, and editing; S.A.: funding acquisition, resources, and supervision. A.D., A.H., R.N.M., and B.N.P.: draft writing, review, and editing; A.D and P.S.: data curation and formal analysis. P.K.S.: resources and supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved and follows the guidelines of the ethical committee of ICAR-Central Institute of Freshwater Aquaculture; all experiments were performed in compliance with the ARRIVE guidelines.
Consent for publication
All data have no human data or results.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling editor: Amany Abbass
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Co-infection of Fusarium incarnatum FHHS2 and Acinetobacter bohemicus FHHK1 was reported for the first time in captive-reared pre-adult Tenualosa ilisha.
• Infected fish exhibited dermatitis, blackening of gills, scale sloughing, and epidermis loss resulting in 40% mortality of hilsa.
• Significant histopathological alterations were observed in the skin, gill, and kidney of infected hilsa.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hoque, F., Adhikari, S., Das, A. et al. Co-infection of Fusarium incarnatum FHHS2 and Acinetobacter bohemicus FHHK1 in captive-reared Hilsa shad, Tenualosa ilisha (Hamilton, 1822). Aquacult Int 32, 1191–1211 (2024). https://doi.org/10.1007/s10499-023-01212-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01212-6