Abstract
Watercress (Nasturtium officinale) has spread widely from Europe and commonly occurs in Oklahoma (USA) springs. Watercress is usually an emergent plant and affects water flow patterns and may provide habitat for biota. Although watercress is not considered an invasive species, its impacts in springs have not been reported. With a goal to describe possible impacts of watercress in springs, 14 karst-associated springs (12 with watercress) were surveyed for sediment characteristics, macroinvertebrates, and diatoms in July 2021. The effects of watercress were evident. Sediment particle size was unaffected by the presence of watercress but sediment organic matter was higher under watercress beds than outside of beds. Although there was no difference in total benthic macroinvertebrate density or richness, higher organic matter was associated with slightly higher abundances of detritivorous and predatory macroinvertebrates (SIMPER). Submerged portions of watercress had significantly much lower diatom density than other spring substrates, with diatom composition similar to other plants but different from that of rocks. Self-shading or possible allelopathy may cause the low diatom density. The significantly lower macroinvertebrate density within watercress mats relative to that of other plants may result from a reduced food source because the plant’s low diatom density. Only predatory damselflies were more common in watercress than in other plants, which had more abundant grazers. Although watercress can increase heterogeneity in sediments and is sometimes valued as an edible plant, watercress supports low algal and macroinvertebrate densities, such that extensive growth of watercress can have an overall negative impact on spring ecosystems.
Similar content being viewed by others
Introduction
Aquatic habitats seem to be especially prone to invasion by plants that can spatially dominate the ecosystem (Hill et al. 2020; Hussner et al. 2021; Strayer 2010). Examples include the submerged plant hydrilla [Hydrilla verticillata (L. f.) Royle)] (Langeland 1996), the floating macrophytes water hyacinth [Eichhornia crassipes (Mart.) Solms] (Villamagna and Murphy 2010), the shoreline/wetland plant purple loosestrife (Lythrum salicaria L.) (Blossey et al. 2001), and the riparian tree salt cedar (Tamarix ramosissima Ledeb.) (Di Tomaso 1998; Stromberg et al. 2009). This study targets a neglected invader–watercress (Nasturtium officinale W.T. Aiton), which is widespread in springs and spring brooks in North America (e.g., Bowles and Bowles 2015; Goerndt et al. 1985; Gooch and Glazier 1991; Johnson 1996; Knysh et al. 2016; Newman et al. 1992; Tenorio and Drezner 2006; Ward and Dufford 1979).
Watercress is a native of Europe and possibly Asia but is now widely spread across the globe. The species occurs in Africa, Australia and New Zealand, and North and South America (Howard and Lyon 1952), including 48 of the 50 states in the USA (USDA 2023). Despite its wide distribution, watercress is seldom listed as a problematic invasive (e.g., CABI 2019; USDA 2022). As an edible plant, watercress was likely introduced to many locales in association to the species cultivation (Howard and Lyon 1952) and has since spread further on its own through both seeds and vegetative reproduction (Howard and Lyon 1952). This dispersal predated widespread concern about the movement of plants and animals to new areas; hence, watercress is considered a naturalized plant. Another possible reason for the apparent lack of concern may be that watercress is less apparent because it tends to have a narrowly defined habitat, including areas of upwelling water, which is found in springs (Johnson 1996) and along some streams (Tarhule and Bergey 2005). Although watercress can certainly be considered invasive based on its spread and formation of large mats, its benefits are usually thought to outweigh possible costs of its invasiveness (CABI 2019)—even though actual impacts have not been investigated (e.g., Hill et al. 2020).
Research on other non-native aquatic plants gives some insight into possible effects that watercress may have on ecosystems. Generally, initial studies of non-native invasive aquatic plants document a number of impacts, but later studies are more inclusive and find some beneficial aspects of some of these plants (Schlaepfer et al. 2011; Stromberg et al. 2009). Invasive plants may physically alter habitats, often with secondary impacts on the biota. Extensive growth can clog waterways, reducing flow rates and increasing sedimentation rates (Blackburn et al. 1982). The presence and architecture of invasive plants may increase habitat complexity, which in turn supports macroinvertebrate communities (Posey et al. 1993; Villamagna and Murphy 2010) and may provide nursery habitat for fish (Evans et al. 2007). Thick mats shade the water column and can alter the diversity of benthic macroinvertebrates and algae (Midgley et al. 2006). As aquatic plants, non-native species may also contribute to ecosystem services, including provisioning services (providing food) and supporting services (sediment formation, primary production and oxygen generation, and providing habitat) (Thomaz 2023).
Displacement of native plant species is a common ecological attribute of invasive plants (Bailey et al. 2001; Langeland 1996; Posey et al. 1993), which also affects animals relying on the displaced plants (Blossey et al. 2001). Invasive species may also provide a new food source (Reynolds 1981), including the use of flowers by pollinators (Anderson 1995).
The goal of this study was to determine some of the effects that watercress might have on spring ecosystems. Unfortunately, watercress was already widespread, so that comparisons of springs before and after colonization or with and without watercress was not possible. Instead, diatom and macroinvertebrate communities associated with watercress were compared with communities associated with other spring habitats, such as other aquatic plants, detritus and benthic rocks. Sediment particle size and organic matter content within and outside beds were assessed, as was the presence of upwelling. Hypotheses tested were: (1) reduction in water velocity within watercress beds will result in finer substrates and more organic material in the bed substrate; (2) watercress will support a richer substrate-dwelling macroinvertebrate community than the surrounding substrate because of the increased sediment organic matter; (3) diatoms will be abundant on submerged watercress stems and leaves; and (4) macroinvertebrates will be common within watercress beds because of added plant architecture and a greater area for periphyton growth.
Materials and methods
Study sites
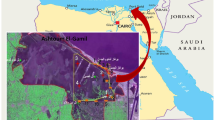
The 14 sampled springs are in the Arbuckle-Simpson Aquifer in south-central Oklahoma (USA) and comprised 9 springs in Johnston County and 5 springs in Pontotoc County (Fig. 1; Table 1). The region is characterized by calcareous limestone and dolomite with outcrops forming the highly eroded Arbuckle Mountains. The studied springs had varied surrounding land use. Two springs were on state wildlife lands, one site was managed by The Nature Conservancy, and the remaining sites were on private property. Springs ranged from heavily impacted (one spring was impounded with trampling by feral hogs) to no apparent impacts (several springs). Nearly all springs had riparian zones with trees and undergrowth. Six springs were in unimproved pasture areas grazed by livestock or used for haying, four springs were formerly grazed but have not been grazed for several years, and three springs were in areas not grazed by livestock. Three springs are partially piped as water sources for households or livestock. These impacts are typical of springs in the region (e.g., Bergey et al. 2008). Descriptions of individual springs are in Online Resource 1 (file ESM_1).
Biota sampling and processing
Watercress at each site was visually scored on a 0–5 scale, with 0 = no watercress found, 1 = scattered single plants, 3 = scattered thin-density beds, and 5 = one or more dense continuous beds.
Substrate-associated macroinvertebrates were sampled using a corer, and macroinvertebrates associated with plants (including watercress) or organic debris (including woody debris) were sampled with a hand net. To accommodate small springs and small plant beds, a 9 cm diameter (about 64 cm2 area) corer was made by cutting the bottom from a plastic jar. The corer was pushed up to 3 cm into the bottom substrate, the lid added, and the core contents lifted up after enclosing the bottom with a metal plate. Paired samples were collected within and outside watercress beds. Core contents were placed in a bucket and sieved to remove fines using a 0.75 mm mesh sieve. Net samples were two short passes through vegetation or across agitated organic debris using an aquarium net frame (width = 20 cm) refitted with a fine mesh (mesh opening = 0.25 × 0.75 mm). Depending on the presence of other species of vegetation, two to three non-replicated cores and net samples were collected per site. Labeled core and net samples were preserved in ethanol. Macroinvertebrates were picked from samples under 10 × magnification using a microscope and preserved in ethanol. Macroinvertebrates were identified to genus for most insects (chironomids were identified to subfamily; small damselfly nymphs were identified to family) and to the lowest easily identified level for non-insects (e.g., genus for snails but not beyond Oligochaeta or Nematoda for these taxa).
Diatoms on surfaces were sampled by collecting submerged vegetation (i.e., watercress and any other plants), wood, and benthic stones, with a single sample containing several pieces of the same type of substrate. Labeled samples were placed in Whirl-paks (Madison, Wisconsin), iced in the field and stored frozen in the laboratory. Samples were thawed and treated with 30% hydrogen peroxide overnight to separate diatoms from substrates. The decanted material was rinsed of hydrogen peroxide through a series of dilutions with water, settling and decanting. All samples were concentrated to a volume of 10 ml and stored refrigerated in capped plastic centrifuge tubes.
Diatom density was measured using a 1 ml subsample from each cleaned 10 ml sample, which was diluted to 10 ml and a 1 ml subsample was placed in a Sedgewick-Rafter counting chamber. The contents of the Sedgewick-Rafter cell were scanned and, if needed, replaced with another subsample that was further diluted or was more concentrated. The resulting sample was viewed at 400 × and the number of diatoms in 15 haphazardly selected fields were counted. The field of view was 2 mm in diameter and the total area of the counting cell was 250 mm2. Raw counts were converted to counts per sample and then counts per mm2 of substrate. Substrate areas were measured using a leaf area scanner. Watercress and other plants were placed in a tray of water and floated onto plastic sheets, which were photocopied and the plant area in these photocopies was measured using a dedicated photo scanner and the WinFolia program (Regent Instruments, Canada). The rock and wood sample areas were measured similarly, except that the photocopies were traced using a light table to avoid shadows counting as area, tracings were colored, and area of each tracing was measured with the leaf area scanner.
To determine diatom taxonomic composition, cleaned subsamples were dried onto coverslips and heated in a muffle furnace at 550 °C, which burned away any remaining organic matter (Zoto et al. 1973). This process was repeated if further dilutions or concentrations were needed to produce a usable dispersion of diatoms on the coverslips. Coverslips were mounted with Naphrax (Brunel Microscopes Ltd, Wiltshire, U.K.), and diatoms were viewed at 1000 × magnification. Diatoms were identified along transects across the coverslip until at least 300 diatoms were identified to genus. Identifications were based on Spaulding et al. (2021). Diatom composition data were converted to relative abundance.
To minimize any observer bias, all samples were labeled with only site codes at collection and data from samples were associated with the site names and locations only after laboratory work was complete. In addition, sediment sample and diatom processing involved moving samples to numbered pre-weighed pans or clean vials, and sample data were re-associated with sample codes and sites after data were collected.
Physical habitat measurements
In addition to macroinvertebrate core samples, separate cores were collected to determine substrate particle size composition and organic matter within and next to watercress beds. These cores were bagged directly after collection (without sieving), iced in the field and stored frozen.
Photosynthetically active radiation (PAR) was measured over watercress beds and in nearby open areas with a handheld PAR meter (SpotOn Quantum PAR Meter; Innoquest, Woodstock, IL, USA), using readings integrated over a 30-s interval. Spring discharge was calculated from a series of water depth and discharge taken with a Marsh-McBriney Flo-mate Meter (Frederick, Maryland). One site had a small waterfall, and discharge was measured as time to fill a bucket of measured volume. The parameters of water temperature, dissolved oxygen, pH and conductivity were measured using a YSI meter and handheld Milwaukee pH probe. GPS coordinates were obtained with a Garmin GPS unit.
Mini-piezometers were used to indicate areas with upwelling and downwelling water within the substrate with respect to watercress bed location. Mini-piezometer design was based on Martinez (2019) but was modified for use in small springs. The clear upright ‘pipe’ was 6.4 mm internal diameter clear flexible plastic tubing, which had 3–4 holes cut in the bottom two cm. The holes and bottom of the tubing were covered with fine mesh that was glued in place with hot glue. The meshed end was hand dug into the substrate to a depth below the top holes in the tubing (e.g., within the root depth of watercress plants). A rubber bulb was used to draw water through the tubing to a level above the water surface before the suction was released and the water level in the tube was allowed to stabilize. Differences between the water levels inside and outside the tubing were recorded. Three to four replicate reading were taken within watercress areas and outside but near watercress plants, and replicate readings were averaged.
Substrate analysis
Frozen samples were thawed and watercress (including roots), and other live vegetation was removed. Much of the organic matter was collected by adding water, swirling, and decanting the lighter-weight organics in a fine (mesh size = 0.5 mm) sieve. The remainder of the sample, consisting primarily of hard substrates, was size portioned by wet sieving through a set of sieves (16, 4, 2, 0.5, and 0.25 mm; and material passing through the 0.25 mm sieve), which corresponded to Wentworth particle size categories ranging from pebbles or larger to fine sand or smaller (Wentworth 1922). All portions were dried at 60 °C, weighted, ashed at 500 °C and reweighed to determine dry weight and ash-free dry weight (AFDW). The mass lost during ashing the organic and particle size substrates were assumed to be organic matter and the ‘ash’ was assumed to be the inorganic substrate.
Statistical analysis
Diatom and macroinvertebrate density and taxonomic richness metrics were analyzed with paired t tests, with paired samples of the various sampled substrates within a spring. Paired sample tests were used because the sampled substrates varied among springs (e.g., not all springs had watercress, other plants–or even rocks). Data sets not meeting the normality assumption were log(x + 1) transformed. Graph and data tables show untransformed data. Analyses were done using SigmaPlot, version 14.5 (Inpixon, Palo Alto, CA, USA).
Diatom and macroinvertebrate composition data were analyzed using PERMANOVA to test for site and substrate differences, using Bray–Curtis similarity matrices and 9999 permutations. Diatom taxonomic counts were standardized to relative abundance because count numbers varied from the 300-count target, and these numbers were not a measure of diatom density. Macroinvertebrate net and core data were log(x + 1) transformed to reduce the effects of low-density taxa. SIMPER was used to indicate differences in the relative abundance of taxa between pairs of substrates. Primer-E and PERMANOVA + (Quest Research Ltd, Auckland, NZ) were used for these analyses.
Sediments were analyzed similarly to the biota data. Organic matter and fine sediment mass in and near watercress beds were compared using paired t tests. Fine sediments were the combined ashed mass of particles passing through a 2 mm mesh (sand and smaller sizes). Particle size composition of sediments inside and outside of watercress beds was compared using PERMANOVA, with samples comprised of the mass of ashed material that was fractionated using sieving (i.e., 16, 4, 2, 0.5, 0.25, < 0.25 mm mesh opening sizes).
Water velocity and water depth were compared between watercress beds and mean spring values using paired t tests. Mean spring values were obtained from velocity and depth measurements taken across a transect as part of measuring spring discharge. Mean mini-piezometer values were compared between watercress beds and areas next to the beds using paired t-teats.
Results
Habitat characteristics
Habitat metrics for each spring are summarized in Table 1 and descriptive accounts of each spring are in Online Resource 1 (file ESM_1). Variation in recorded water temperatures (median = 18.1 °C) and dissolved oxygen (median = 4.6 ppm) among springs was relatively high because of spring modifications, especially spring excavation or damming, and watercress occurring in the springbrook and not the springhead. For example, Houghtubby Spring was excavated to form a pond (with cattails and wild hog disturbance), and watercress was found in the overflow springbrook, resulting in an elevated water temperature of 28.4 °C at the watercress site. Water pH averaged 7.4 (SE = 0.1) and conductivity averaged 546.1 (SE = 11.1) μS/cm–excluding Viola Spring’s high measurement of 1966 μS/cm. Calculated discharge varied greatly among sites, ranging from 0.09 to 156.07 l/s.
Watercress beds
Watercress varied from small isolated plants with reduced leaf sizes to large dense beds. Watercress scores were fairly evenly represented across the 0–5 scale (Table 1). Water depth in watercress beds was not significantly different from the mean depth (based on depth transects used for discharge calculations) (Table 2; Fig. 2a). Watercress beds were associated with lower water velocities than the average spring velocity (p = 0.0073; Table 2; Fig. 2b), based on water velocity transects.
Habitat comparisons between watercress beds and mean spring values (= Spring) or the substrate outside of watercress beds (= Outside). Bars indicate + 1 SE and different letters over bars indicate statistically significance differences (Table 2)
Mini-piezometers demonstrated upwelling in 10 of the 12 watercress beds (Table 1). The two springs without measured upwelling in watercress were two of the springs where watercress occurred downstream of the springhead. There was no significant difference in upwelling between watercress beds and nearby areas outside of beds (Table 2; Fig. 2c).
PAR light levels directly above watercress beds (Table 1) averaged 203.2 μmol m−2 s−1 (SE = 64.2; N = 12) whereas springs lacking watercress averaged 13.7 μmol m−2 s−1 (SE = 0.1; N = 2). Most springs were well shaded by riparian vegetation and PAR levels in nearby unshaded areas averaged 1275.9 μmol m−2 s−1 (SE = 115.6; N = 9). Springs with PAR levels below approximately 75 μmol m−2 s−1 had low (0–3) watercress scores (Fig. 3). A reading of 75 μmol m−2 s−1 is 5.5% of the mean unshaded ambient light level.
Sediments
Sediment particle size composition differed among spring sites (p = 0.0026; Table 2), with sediments ranging from muddy-silt to bedrock with interspersed fine material. Sediment size composition did not differ between sediments in watercress beds and sediments near the beds (p = 0.1082; Tables 2 and 3).
Organic matter biomass in sediments below watercress beds was significantly higher than organic matter in sediments outside watercress beds (p = 0.0031; Table 2, Fig. 4), with over twice as much organic matter in watercress bed sediments than sediment outside of the watercress beds (means (SE) = 7.11 (2.47) and 2.80 (0.71) g/sample, respectively). In contrast to organic matter content, fine sediment weight did not differ between sediments in watercress beds and nearby sediments (p = 0.849; Table 2, Fig. 4).
Organic and fine sediments beneath and next to watercress beds. Bars indicate + 1 SE and different letters over bars indicate statistically significance differences (Table 2)
Diatoms
Watercress had significantly lower diatom density than both rocks collected near the watercress beds and other nearby organic substrates in the spring (Table 2, Fig. 5a). Although rocks had a lower mean diatom density than other substrates, the variation in density among the other substrates was large, resulting in a p value near 0.05 (p = 0.056). Diatom density on watercress averaged 631 diatoms/mm2; rocks averaged 5,949 diatoms/mm2, and other organic substrates averaged 24,457 diatoms/mm2. Among these other substrates, Myriophyllum averaged 59,087 diatoms/mm2, whereas averages for moss and wood were lower, at 14,188 and 87 diatoms/mm2, respectively (Fig. 5a).
Diatom density and taxonomic richness on three types of spring substrates: submerged watercress leaves, rocks, and ‘Other’, which includes other plants and detritus. The ‘Other’ category for diatom density includes the mean values for 3 substrates indicated (each with N = 4). Bars indicate + 1 SE and different letters over bars indicate statistically significance differences (Table 2)
Diatom taxonomic richness did not differ among watercress, rock, and other substrates (Table 2; Fig. 5b). In contrast, diatom taxonomic composition differed among substrates (watercress, rocks, other organic substrates; p = 0.0167), and among springs (p = 0.0113). The substrates–springs interaction was non-significant (p = 0.987). Details of the taxonomic composition of the samples in each spring are in the associated datasets (Bergey 2022). Based on SIMPER results (Table 4), watercress had higher abundances of Cocconeis, Meridion and Eunotia and rocks had more Achnanthidium and Amphora relative to the other substrate types. The other organic substrates were somewhat intermediate, with slightly lower abundance of Cocconeis, Meridion and Eunotia than watercress and an intermediate abundance of Achnanthidium between rocks and watercress.
Macroinvertebrates
Macroinvertebrates collected by netting were those in plants, and leaf and woody detritus rather than within the bed substrate. Macroinvertebrate density was almost four times higher in other aquatic plants than in watercress (p = 0.0314; Table 2 and Fig. 6a), but was not significantly different between watercress and detritus (p = 0.578). The paired comparison between other plants and detritus had only three replicates, so results may not be representative (p = 0.652). Taxonomic richness did not differ among the substrates (Table 2; Fig. 6b).
Macroinvertebrate density and richness of watercress beds and other nearby substrates. Netted macroinvertebrates were captured by sweeping a net through possible substrates (a = density; b = richness). Core invertebrates were captured using a shallow core and include surface-dwelling and within-sediment invertebrates (c = density; d = richness). Bars indicate + 1 SE and different letters over bars indicate statistically significance differences (Table 2)
The first 12 taxa identified by each of the three pairwise combinations using SIMPER corresponded closely with the overall 12 most abundant taxa (= taxa with 100 or more collected individuals; Table 5). Watercress had the highest density of early instar damselflies (Coenagrionidae) and ostracods, other plants had high densities of the amphipod Hyalella, the mayflies Fallceon and Callibaetis, Physa snails, and cyclopoid copepods. Other substrates (detritus) had highest densities of planarians, chironomids midge larvae (Chironominae, Orthocladinae, and Tanypodinae) and the caddisfly Helicopsyche.
The density of sediment-dwelling macroinvertebrates (number per 64 cm2 sample) did not differ in the sediments below watercress beds and the sediments outside beds (p = 0.882, Table 2), averaging 97.6 and 103.5 individuals, respectively (Fig. 6c). Taxonomic richness also did not differ within and outside of watercress beds (p = 0.111), averaging 9.4 and 7.1 taxa, respectively (Fig. 6d).
Taxonomic composition of macroinvertebrates associated with the sediment differed among spring sites but was not significantly different between watercress and outside areas (Table 2). Details of the taxonomic composition of samples in each spring are in the associated datasets (Bergey 2022). Although the overall composition inside and outside of watercress beds was not significantly different, SIMPER analysis indicated interesting occurrence patterns (Table 5). Watercress sediments had higher densities of some substrate-dwelling macroinvertebrates (oligochaete worms, the fingernail clam Sphaerium, and nematodes), whereas non-burrowers (coenagrionid damselflies, the mayfly Baetis, and the amphipod Hyalella) were more commonly associated with sediments outside of watercress beds (Table 6).
Discussion
Plant beds in flowing water normally accrue fine sediment because of the reduced water velocity within beds (Gallardo et al. 2016; Rovira et al. 2016); however, substrates beneath watercress beds accrued organic matter but not fine mineral sediments. The lower than ‘expected’ fine sediment is likely the result of the nature of springs. Rather than carrying a sediment load from upstream, the groundwater source in springs is nearly sediment free (Ward and Dufford 1979). Hence, the flowing water in springs is not simultaneously dropping and picking up sediment but is primarily picking up fine sediment, which results in the lack of accumulation of fine sediment within springs. Upwelling water flow within sediments (including within and next to the sampled watercress beds) likely also reduces the settlement of fine sediments. Low sediment accumulation minimizes the adhesion within fine sediments, further increasing the likelihood of entrainment of any settled fine sediments (Nafchi et al. 2021). Organic matter moving downstream would act similarly because the groundwater source is also very low in organic matter (Ward and Dufford 1979), indicating that the higher organic matter within than outside beds is produced in situ, presumably from decaying matter from the watercress plants along with possible entrapment of leaves and other organic material (Glazier 1991), especially given the extensive riparian trees at most of the sites. Organic matter in the form of invertebrate-derived detritus and resting stages may also accrue in sediments under plant beds (Rodrigo et al. 2021).
This elevated organic matter under watercress beds compared to areas next to beds was reflected in the macroinvertebrate taxonomic composition beneath the watercress beds and contributes to habitat heterogeneity in springs. No taxa were found exclusively under the beds, but the under-bed fauna had more detritivorous taxa than the nearby exposed bed. These included oligochaetes, orthoclad chironomids, and fingernail clams. Although all three groups contain species with varied diets, most are associated detritus and the accompanying microbial communities (Coffman and Ferrington Jr. 1996; Marshall 1973). Nematodes and tanypod chironomids were also more abundant under watercress, and these groups includes many predators of small invertebrates. In addition to higher organic matter, the roots of watercress stabilize the sediments, which might also improve the habitat for some macroinvertebrates, such as fingernail clams.
Watercress had a much lower density of diatoms than did the other categories of substrates; only wood within the combined plants and detritus category had such a low density. The low wood value is explained, in part, because two of the four sites with wood were the two sites with no watercress, moss or any other higher plants, presumably because of the heavy shade by the dense riparian cover–shade that would also reduce diatom densities. In the field, watercress plants, including submerged leaves, were bright green, whereas other plants were tinted and fuzzy because of a high periphyton cover. Despite the low diatom density, taxonomic composition was similar between watercress and the plant/detritus categories. Diatom composition differed between rocks and samples taken from plants; a pattern also found by Round (1957) in springs in the United Kingdom. Watercress and plants/detritus had similar compositions, with watercress having more Meridion (all were Meridion circulare) and Eunotia spp. than the combined other plants/detritus.
The low density of diatoms on watercress is associated with at least three possible factors. First, because watercress typically grew in areas of upwelling groundwater, this direct contact with groundwater could influence the diatoms on watercress more than on other spring substrates. However, the substrates near watercress beds also had upwelling but the rocks did not have this reduced diatom density. Instead, the exposure to upwelling groundwater may account for the higher relative abundance of Meridion and Eunotia on watercress. A second reason could be low light levels on submerged watercress leaves because of self-shading by the emergent parts of the plants, which were often also shaded by riparian vegetation. Light level cannot be excluded as a cause because watercress is tolerant of low light levels (this study), growing at levels as low as 7% of ambient light (Going et al. 2008) and shade by the emergent potions of beds would further shade the periphyton. Submerged leaves were collected from the edges of beds, so although low light levels likely impacted diatom density, the presence of angled and scattered light at the edges of beds makes it unlikely that low light levels are the sole cause of the low diatom density. A third possibility are the defense chemicals in watercress. The characteristic flavor of watercress results from 2-phenylethyl isothiocyanate, which is an herbivore defense chemical released from damaged tissue (Newman et al. 1992). This chemical is produced when tissue disruption releases the precursor (a glucosinulate) and enzyme (myrosinase) and is not normally present in undamaged tissues (Newman et al. 1992). Watercress isothiocyanate is toxic to amphipods (Newman et al. 1990) but not aquatic insects or snails (Newman et al. 1996). In a stream with watercress and the macroalga Cladophora sp., macroinvertebrates ate periphyton, Cladophora and plant detritus, but not live watercress (Koslucher and Minshall 1973), whereas stable isotope analysis of the food webs in three springs indicated that watercress was, indeed, consumed (Carroll et al. 2016). However, the watercress may have been consumed as detritus (Carroll et al. 2016; Koslucher and Minshall 1973), which is no longer chemically defended (Newman et al. 1996). Yellowed or decayed leaves were not apparent in the sampled springs, which is consistent with their consumption by macroinvertebrates. The potential of watercress allelopathy against diatoms or other autotrophs is untested, but allelopathy is a trait that can impact other aquatic autotrophs (Schultz and Dibble 2012), including epiphytes (Wijewanrene et al. 2022). Allelopathy has been found in several aquatic macrophytes (Gross 2003; Wijewanrene et al. 2022), and impacts on phytoplankton (Rojo et al. 2013) are more evident than on epiphytes (Hilt and Gross 2008).
Although the watercress beds may not provide a major periphyton food source, watercress has an architecture that provides shelter (Glazier 1991), but this within-bed habitat is little used by macroinvertebrates, whereas other non-native macrophytes often increase macroinvertebrates populations (reviewed by Tasker et al. 2022). Macroinvertebrates were abundant in all sampled substrates, and density was similar between detritus and watercress but lower in watercress than in other plants (e.g., Myriophyllum, moss). This lower density of macroinvertebrates corresponds with the low diatom density in watercress. One taxon that was relatively more abundant in watercress was damselfly nymphs, which are clinging predators for which the architecture of watercress provides an appropriate habitat.
Victor Shelford (1918) is frequently quoted for his concept of the ecosystem relationship of aquatic plants, specifically "One could probably remove all the larger plants and substitute glass structures of the same form and surface texture without greatly affecting the immediate food relations" (e.g., O’Hare et al. 2018). This is taken somewhat out of context because in the same paragraph, Shelford mentions the periphyton on plants as a food source for invertebrates and the architecture of plants proving shelter. Based on the low diatom density and resultant lower than expected macroinvertebrate density within the beds, Shelford’s generalization may pertain to watercress, as glass would greatly diminish self-shading and not have any possible defense/allelopathic effects; however, glass plants may not supply detritus to the food web or to the organic matter in the springbed (not to mention hazards posed by broken glass).
The overall effects of watercress on diatom and macroinvertebrate communities are neutral to slightly negative, but there are other considerations to the value of watercress beyond this study. Although watercress can form dense beds, there are no reports that the species has crowded out any native species. Where watercress occurs in high densities, water flow can be impeded, resulting in greater wetted area (Cushing and Wolf 1984). However, removal of watercress can also impact springs (Gooch and Glazier 1991). Yellow, senescent leaves are no longer chemically defended and are eaten by several aquatic invertebrates (Kerfoot et al. 1998; Newman et al. 1992), and watercress contributes to the diet of a few terrestrial herbivores—including specialized chrysomelid beetles (Gray et al. 1983) and generalists such as wild turkeys (Goerndt et al. 1985). Additionally, watercress is consumed by humans, who have been an important vector in introducing and spreading the plant (Blüthner 2020; Green 1962). Some of this consumption is based on watercress’s flavor, nutrient content, and its medicinal value (Blüthner 2020).
This study targeted the ecosystem effects of watercress but ecosystem effects on watercress were also apparent. For example, low light level impacted plant height and bed size, as well as watercress presence. Other watercress studies have documented morphological or density effects of light level (Going et al. 2008; Knysh et al. 2016) and spring aspect, size and water flow (Tenorio and Drezner 2006); factors that likely contributed to the morphological variation observed among watercress populations by Jafari and Hassandokht (2012). A recent method for measuring stress in aquatic plants is using tissue peroxide (H2O2) concentration (Asaeda et al. 2023). This measurement may be an especially suitable metric for investigating the relationship between stress and the varied morphology of watercress because H2O2 levels may be affected by light intensity, water temperature, and flow velocity (Asaeda et al. 2021), factors that can also affect watercress morphology. It may also be possible to test H2O2 levels of epiphytes on watercress versus on other plants, as this metric has been used on cyanobacteria (Asaeda et al. 2022).
Conclusions
Of the four tested hypotheses, two were partly supported (watercress accrued organic matter but not fine mineral sediments, and substrate-dwelling macroinvertebrate communities under watercress beds were diverse but were not more taxa-rich than outside watercress beds). Two hypotheses were not supported: diatoms were much less abundant on submerged watercress leaves than on other spring substrates, and watercress had lower macroinvertebrate density than did other plants.
Watercress roots in areas of minor upwelling where other rooted plants do not normally grow. Within sediments, organic matter accrual beneath watercress plus substrate stability by roots enables a community with more detritivores than areas without watercress—a pattern that increases spatial heterogeneity within springs. The low density of diatoms on watercress indicates barriers to growth—possibilities include low light levels from self-shading and allelopathy. Low macroinvertebrate density is consistent with low food (diatom) availability and anti-herbivore chemical defenses, although the presence of damselfly nymphs indicates habitat use by these predators. The question of possible allelopathy by watercress remains and is deserving of study.
Data availability
References
Anderson MG (1995) Interactions between Lythrum salicaria and native organisms: a critical review. Environ Manag 19:225–231. https://doi.org/10.1007/BF02471992
Asaeda T, Rahman M, Abeynayaka HDL (2022) Hydrogen peroxide can be a plausible biomarker in cyanobacterial bloom treatment. Sci Rep 12:12. https://doi.org/10.1038/s41598-021-02978-6
Asaeda T, Rashid MH, Liping X et al (2023) The distribution of submerged macrophytes in response to intense solar radiation and salinity reveals hydrogen peroxide as an abiotic stress indicator. Sci Rep 13:4548. https://doi.org/10.1038/s41598-023-30487-1
Asaeda T, Rashid MH, Schoelynck J (2021) Tissue hydrogen peroxide concentration can explain the invasiveness of aquatic macrophytes: a modeling perspective. Front Environ Sci. https://doi.org/10.3389/fenvs.2020.516301
Bailey JK, Schweitzer JA, Whitham TG (2001) Salt cedar negatively affects biodiversity of aquatic macroinvertebrates. Wetlands 21:442–447. https://doi.org/10.1672/0277-5212(2001)021[0442:SCNABO]2.0.CO;2
Bergey EA, Matthews WJ, Fry JE (2008) Springs in time: fish fauna and habitat changes in springs over a 20-year interval. Aquat Conserv Mar Freshw Ecosyst 18:829–838. https://doi.org/10.1002/aqc.893
Bergey EA (2022) Watercress springs survey datasets. Open science framework (OSF) https://doi.org/10.17605/OSF.IO/GZ5UH
Blackburn WH, Knight RW, Schuster JL (1982) Saltcedar influence on sedimentation in the Brazos River. J Soil Water Conserv 37:298–301
Blossey B, Skinner LC, Taylor J (2001) Impact and management of purple loosestrife (Lythrum salicaria) in North America. Biodivers Conserv 10:1787–1807. https://doi.org/10.1023/A:1012065703604
Blüthner W-D (2020) Nasturtium officinale R.Br.: watercress. In: Novak J, Blüthner W-D (eds) Medicinal, aromatic and stimulant plants. Springer International Publishing, Cham, pp 333–344
Bowles DE, Bowles BD (2015) Non-native species of the major spring systems of Texas, USA. Tex J Sci 67:51–78
CABI (2019) Nasturtium officinale (watercress) datasheet. In: CAB International. https://www.cabi.org/isc/datasheet/35646 Accessed: 14 Oct 2022 2022
Carroll TM, Thorp JH, Roach KA (2016) Autochthony in karst spring food webs. Hydrobiologia 776:173–191. https://doi.org/10.1007/s10750-016-2750-6
Coffman WP, Ferrington LC Jr (1996) Chironomidae. In: Merritt RW, Cummins KW (eds) An introduction to the aquatic insects of North America, 3rd edn. Kendall/Hunt Publishing Company, Dubuque, Iowa, pp 635–748
Cushing CE, Wolf EG (1984) Primary production in Rattlesnake Springs, a cold desert spring-stream. Hydrobiologia 114:229–236. https://doi.org/10.1007/BF00031874
Di Tomaso JM (1998) Impact, biology, and ecology of saltcedar (Tamarix spp.) in the southwestern United States. Weed Technol 12:326–336. https://doi.org/10.1017/S0890037X00043906
Evans JM, Wilkie AC, Burkhardt J, Haynes RP (2007) Rethinking exotic plants: using citizen observations in a restoration proposal for Kings Bay, Florida. Ecol Restor 25:199–210. https://doi.org/10.3368/er.25.3.199
Gallardo B, Clavero M, Sánchez MI, Vilà M (2016) Global ecological impacts of invasive species in aquatic ecosystems. Glob Change Biol 22:151–163. https://doi.org/10.1111/gcb.13004
Glazier DS (1991) The fauna of North American temperate cold springs: patterns and hypotheses. Freshw Biol 26:527–542. https://doi.org/10.1111/j.1365-2427.1991.tb01417.x
Goerndt DL, Schemnitz SD, Zeedyk WD (1985) Managing common watercress and spring/seeps for Merriam’s turkey in New Mexico. Wild Soc Bull 13:297–301
Going B, Simpson J, Even T (2008) The influence of light on the growth of watercress (Nasturtium officinale R. Br.). Hydrobiologia 607:75–85. https://doi.org/10.3368/er.25.3.19910.1007/s10750-008-9368-2
Gooch JL, Glazier DS (1991) Temporal and spatial patterns in mid-Appalachian springs. Mem Entomol Soc Can 123:29–49. https://doi.org/10.3368/er.25.3.19910.4039/entm123155029-1
Gray LJ, Ward JV, Martinson R, Bergey E (1983) Aquatic macroinverterbrates of the Piceance Basin, Colorado: community response along spatial and temporal gradients of environmental conditions. Southwest Nat. https://doi.org/10.2307/3671380
Green PS (1962) Watercress in the new world. Rhodora 64:32–43
Gross EM (2003) Allelopathy of aquatic autotrophs. Crit Rev Plant Sci 22:313–339. https://doi.org/10.1080/713610859
Hill MP, Coetzee JA, Martin GD, Smith R, Strange EF (2020) Invasive alien aquatic plants in South African freshwater ecosystems. In: van Wilgen BW, Measey J, Richardson DM, Wilson JR, Zengeya TA (eds) Biological Invasions in South Africa. Springer, pp 97–114
Hilt S, Gross EM (2008) Can allelopathically active submerged macrophytes stabilise clear-water states in shallow lakes? Basic Appl Ecol 9:422–432. https://doi.org/10.1016/j.baae.2007.04.003
Howard H, Lyon A (1952) Nasturtium officinale R. Br. (Rorippa Nasturtium-Aquaticum (L.) Hayek). J Ecol 40:228–245
Hussner A, Heidbüchel P, Coetzee J, Gross EM (2021) From introduction to nuisance growth: a review of traits of alien aquatic plants which contribute to their invasiveness. Hydrobiologia 848:2119–2151. https://doi.org/10.1007/s10750-020-04463-z
Jafari S, Hassandokht M (2012) Evaluation of some Iranian watercress (Nasturtium officinale L.) populations using agro-morphological traits. Int J For Soil Eros 2:119–123
Johnson GC (1996) A seepage investigation of an area at and near Oak Ridge National Laboratory, Oak Ridge, Tennessee, March through August 1993. US Geological Survey, pp. 51 pp
Kerfoot WC, Newman RM, Hanscom Z (1998) Snail reaction to watercress leaf tissues: reinterpretation of a mutualistic ‘alarm’ hypothesis. Freshw Biol 40:201–213. https://doi.org/10.1046/j.1365-2427.1998.00334.x
Knysh KM, Giberson DJ, van den Heuvel MR (2016) The influence of agricultural land-use on plant and macroinvertebrate communities in springs. Limnol Oceanogr 61:518–530. https://doi.org/10.1002/lno.10230
Koslucher DG, Minshall GW (1973) Food habits of some benthic invertebrates in a northern cool-desert stream (Deep Creek, Curlew Valley, Idaho-Utah). Trans Am Microsc Soc 92:441–452. https://doi.org/10.2307/3225248
Langeland KA (1996) Hydrilla verticillata (L.F.) Royle (Hydrocharitaceae), “the perfect aquatic weed.” Castanea 61:293–304
Marshall JW (1973) A benthic study of the Avon spring stream, Christchurch. Mauri Ora 1:79–90
Martinez CJ (2019) Mini-piezometers for measuring groundwater to surface water exchange. In: University of Florida IFAS Extension Online. Publication #AE454. https://edis.ifas.ufl.edu/ae454 Accessed: March 7, 2023
Midgley JM, Hill MP, Villet MH (2006) The effect of water hyacinth, Eichhornia crassipes (Martius) Solms-Laubach (Pontederiaceae), on benthic biodiversity in two impoundments on the New Year’s River, South Africa. Afr J Aquat Sci 31:25–30. https://doi.org/10.2989/16085910609503868
Nafchi RF, Samadi-Boroujeni H, Vanani HR, Ostad-Ali-Askari K, Brojeni MK (2021) Laboratory investigation on erosion threshold shear stress of cohesive sediment in Karkheh Dam. Environ Earth Sci 80:681. https://doi.org/10.1007/s12665-021-09984-x
Newman RM, Hanscom Z, Kerfoot WC (1992) The watercress glucosinolate-myrosinase system: a feeding deterrent to caddisflies, snails and amphipods. Oecologia 92:1–7. https://doi.org/10.1007/BF00317255
Newman RM, Kerfoot WC, Hanscom Z (1990) Watercress and amphipods: potential chemical defense in a spring stream macrophyte. J Chem Ecol 16:245–259. https://doi.org/10.1007/BF01021282
Newman RM, Kerfoot WC, Hanscom Z (1996) Watercress allelochemical defends high-nitrogen foliage against consumption: effects on freshwater invertebrate herbivores. Ecology 77:2312–2323. https://doi.org/10.2307/2265733
O’Hare MT, Aguiar FC, Asaeda T et al (2018) Plants in aquatic ecosystems: current trends and future directions. Hydrobiologia 812:1–11. https://doi.org/10.1007/s10750-017-3190-7
Posey MH, Wigand C, Stevenson JC (1993) Effects of an introduced aquatic plant, Hydrilla verticillata, on benthic communities in the upper Chesapeake Bay. Estuarine Coastal Shelf Sci 37:539–555. https://doi.org/10.1006/ecss.1993.1072
Reynolds JE (1981) Behavior patterns in the West Indian manatee, with emphasis on feeding and diving. Fla Sci 44:233–242
Rodrigo MA, Puche E, Segura M et al (2021) Sediment underneath charophyte meadows is enriched in viable ephippia and enhances the benthic periphytic biofilm. Hydrobiologia 848:5203–5221. https://doi.org/10.1007/s10750-021-04702-x
Rojo C, Segura M, Rodrigo MA (2013) The allelopathic capacity of submerged macrophytes shapes the microalgal assemblages from a recently restored coastal wetland. Ecol Eng 58:149–155. https://doi.org/10.1016/j.ecoleng.2013.06.019
Round FE (1957) A note on some diatom communities in calcareous springs and streams. Bot J Linn Soc 55:662–668. https://doi.org/10.1111/j.1095-8339.1957.tb00029.x
Rovira A, Alcaraz C, Trobajo R (2016) Effects of plant architecture and water velocity on sediment retention by submerged macrophytes. Freshw Biol 61:758–768. https://doi.org/10.1111/fwb.12746
Schlaepfer MA, Sax DF, Olden JD (2011) The potential conservation value of non-native species. Conserv Biol 25:428–437. https://doi.org/10.1111/j.1523-1739.2010.01646.x
Schultz R, Dibble E (2012) Effects of invasive macrophytes on freshwater fish and macroinvertebrate communities: the role of invasive plant traits. Hydrobiologia 684:1–14. https://doi.org/10.1007/s10750-011-0978-8
Shelford VE (1918) Conditions of existance. In: Ward HB, Whipple GC (eds) Fresh-water biology. Wiley, Hoboken, pp 21–60
Spaulding SA, Potapova MG, Bishop IW, Lee SS, Gasperak TS, Jovanoska E, Furey PC, Edlund MB (2021) Diatoms.org: supporting taxonomists, connecting communities. Diatom Res 36:291–304. https://doi.org/10.1080/0269249X.2021.2006790
Strayer DL (2010) Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshw Biol 55:152–174. https://doi.org/10.1111/j.1365-2427.2009.02380.x
Stromberg JC, Chew MK, Nagler PL, Glenn EP (2009) Changing perceptions of change: the role of scientists in Tamarix and river management. Restor Ecol 17:177–186. https://doi.org/10.1111/j.1526-100X.2008.00514.x
Tarhule AA, Bergey EA (2005) Springs in time: comparison of present and historical flows. Oklahoma Water Resources Center. pp 68
Tasker SJL, Foggo A, Bilton DT (2022) Quantifying the ecological impacts of alien aquatic macrophytes: a global meta-analysis of effects on fish, macroinvertebrate and macrophyte assemblages. Freshw Biol 67:1847–1860. https://doi.org/10.1111/fwb.13985
Tenorio RC, Drezner TD (2006) Native and invasive vegetation of karst springs in Wisconsin’s Driftless area. Hydrobiologia 568:499–505. https://doi.org/10.1007/s10750-006-0106-3
Thomaz SM (2023) Ecosystem services provided by freshwater macrophytes. Hydrobiologia 850:2757–2777. https://doi.org/10.1007/s10750-021-04739-y
USDA (2022) National invasive species information center: aquatic plants. In https://www.invasivespeciesinfo.gov/aquatic/plants Accessed: October 25, 2022
USDA (2023) Nasturtium officinale W.T. Aiton. In. https://plants.usda.gov/home/plantProfile?symbol=NAOF Accessed: August 8, 2023
Villamagna AM, Murphy BR (2010) Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): a review. Freshw Biol 55:282–298. https://doi.org/10.1111/j.1365-2427.2009.02294.x
Ward JV, Dufford RG (1979) Longitudinal and seasonal distribution of macroinvertebrates and epilithic algae in a Colorado springbrook-pond system. Archiv Hydrobiol 86:284–321
Wentworth CK (1922) A scale of grade and class terms for clastic sediments. J Geol 30:377–392. https://doi.org/10.1086/622910
Wijewardene L, Wu N, Fohrer N et al (2022) Epiphytic biofilms in freshwater and interactions with macrophytes: current understanding and future directions. Aquat Bot 176:103467. https://doi.org/10.1016/j.aquabot.2021.103467
Zoto GA, Dillon DO, Schlichting HE (1973) A rapid method for clearing diatoms for taxonomic andecological studies. Phycologia 12:69–70. https://doi.org/10.2216/i0031-8884-12-1-69.1
Acknowledgements
I greatly appreciate the landowners and managers who allowed access to springs, including help in accessing the more remote sites. Eric Bright helped locate potential spring sites to include in this study. Lara Sousa helped with leaf area measurements. Discussions with Kambridge Stephens were fruitful. The University of Oklahoma and Oklahoma Biological Survey funded research travel, conference attendance, and publication funding.
Funding
Funding was provided by the University of Oklahoma through the Sponsored Research Incentive program and an internal grant from the Oklahoma Biological Survey.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has no conflicts of interest to declare.
Additional information
Handling editor: Asaeda Takashi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bergey, E.A. The impacts of non-native watercress in Oklahoma spring ecosystems. Aquat Ecol (2024). https://doi.org/10.1007/s10452-023-10081-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10452-023-10081-3