Abstract
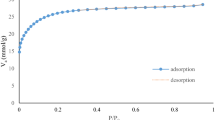
Biobutanol has attracted significant interest in recent decades and is seriously considered as a potential biofuel to partly replace gasoline. However, some production challenges must be addressed to make butanol economically viable such as the low product concentration and product toxicity inhibiting the microorganism. To alleviate these limitations, several in situ or ex situ separation techniques have been investigated in view of their integration to the biobutanol production process to enhance its economic viability. One of these techniques is adsorption which is one of the most energy-efficient techniques used for biobutanol separation. Considering the number of chemical species present in the ABE fermentation broth, it is essential to develop multicomponent adsorption isotherms for all components as a first step to design a high performance adsorption process. Few multicomponent isotherm models have been proposed such as multicomponent Langmuir and Freundlich. In this study, these two models as well as artificial neural networks were used to model the isotherms of each component in an ABE fermentation broth as a function of the equilibrium concentrations of all components for activated carbon F-400. Results showed that the multicomponent Langmuir model was not accurate due to the many simplifying assumptions. The multicomponent Freundlich and feedforward neural network (FFNN) isotherm models were able to predict the behavior of multicomponent systems very well. Indeed, the predictive model of the experimental data had a coefficient of determination (R2) of 0.97 and 0.99, for multicomponent Freundlich and FFNN isotherm models, respectively.






Similar content being viewed by others
Abbreviations
- ABE:
-
Acetone–butanol–ethanol
- ANN:
-
Artificial neural network
- FFNN:
-
Feed forward neural network
- HL:
-
High level
- LL:
-
Low level
- SC:
-
Single component
- a ij :
-
Competition coefficient in multicomponent Freundlich isotherm model
- b :
-
Constant in multicomponent Langmuir isotherm model (L/g adsorbate)
- C :
-
Adsorbate concentration at equilibrium (g/L)
- \(\overline{C}\) :
-
Normalized adsorbate concentration at equilibrium (g/L)
- C*:
-
Adsorbate concentration in equilibrium with adsorbed phase concentration q (g/L)
- n :
-
Constant in multicomponent Freundlich isotherm model
- K :
-
Constant in multicomponent Freundlich isotherm model (L/g adsorbent)
- q :
-
Adsorption capacity (g adsorbate/g adsorbent)
- q*:
-
Adsorption capacity in equilibrium with bulk liquid concentration C (g adsorbate/g adsorbent)
- q s :
-
Saturation adsorption capacity (g adsorbate/g adsorbent)
- \(\overline{q}\) :
-
Normalized adsorption capacity (g adsorbate/g adsorbent)
- W :
-
Weights in FFNN model
References
Abdehagh, N., Tezel, F.H., Thibault, J.: Adsorbent screening for biobutanol separation by adsorption: kinetics, isotherms and competitive effect of other compounds. Adsorption 19, 1263–1272 (2013)
Abdehagh, N., Tezel, F.H., Thibault, J.: Separation techniques in butanol production: challenges and developments (review). Biomass Bioenergy 60, 222–246 (2014)
Abdehagh, N., Gurnani, P., Tezel, F.H., Thibault, J.: Adsorptive separation and recovery of biobutanol from ABE model solutions. Adsorption 21, 185–194 (2015)
Antoni, D., Zverlov, V.V., Schwarz, W.H.: Biofuels from microbes (mini review). Appl. Microbiol. Biotechnol. 77, 23–35 (2007)
Basu, S., Henshaw, P.F., Biswas, N., Kwan, H.K.: Prediction of gas phase adsorption isotherms using neural nets. Can. J. Chem. Eng. 80, 1–7 (2002)
Bulsari, A.B., Palosaafi, A.: Application of neural networks for system identification of an adsorption column. Neural Comput. Appl. 1, 160–165 (1993)
Carsky, M., Do, D.D.: Neural network modeling of adsorption of binary vapour mixtures. Adsorption 5, 183–192 (1999)
Dellomonaco, C., Fava, F., Gonzalez, R.: The path to next generation biofuels: successes and challenges in the era of synthetic biology. Microb. Cell Fact. 9, 3 (2010)
Do, D.D.: Adsorption Analysis: Equilibria and Kinetics. Imperial College Press, London (1998)
Dürre, P.: Biobutanol: an attractive biofuel. Biotechnol. J. 2, 1525–1534 (2007)
Ezeji, T.C., Qureshi, N., Blaschek, H.P.: Production of acetone, butanol and ethanol by Clostridium beijerinckii BA101 and in situ recovery by gas stripping. World J. Microbiol. Biotechnol. 19, 595–603 (2003)
Ezeji, T.C., Qureshi, N., Blaschek, H.P.: Butanol fermentation research: upstream and downstream manipulations. Chem. Rec. 4, 305–314 (2004)
Ezeji, T.C., Qureshi, N., Blaschek, H.P.: Bioproduction of butanol from biomass: from genes to bioreactors. Curr. Opin. Biotechnol. 18, 220–227 (2007)
Fouad, E.A., Feng, X.: Use of pervaporation to separate butanol from dilutes aqueous solutions: effects of operating conditions and concentration polarization. J. Membr. Sci. 323, 428–435 (2008)
Freundlich, H.M.F.: Über die adsorption in Lösungen. Z. Phys. Chem. 57(A), 385–470 (1906)
Groot, W.J., Luyben, K.Ch.A.M.: In situ product recovery by adsorption in the butanol/isopropanol batch fermentation. Appl. Microbiol. Biotechnol. 25, 29–31 (1986)
Harvey, B.G., Meylemans, H.A.: The role of butanol in the development of sustainable fuel technologies. J. Chem. Technol. Biotechnol. 86, 2–9 (2011)
Holtzapple, M.T., Brown, R.F.: Conceptual design for a process to recover volatile solutes from aqueous solutions using silicalite. Sep. Technol. 4, 213–229 (1995)
Jiao, P., Wu, J., Zhou, J., Yang, P., Zhuang, W., Chen, Y., Zhu, C., Guo, T., Ying, H.: Mathematical modeling of the competitive sorption dynamics of acetone–butanol–ethanol on KA-I resin in a fixed-bed column. Adsorption 21, 165–176 (2015)
Langmuir, I.: The adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc. 40, 1361–1402 (1918)
Lewandowski, J., Lemcoff, N.O., Palosaari, S.: Use of neural networks in the simulation and optimization of pressure swing adsorption processes. Chem. Eng. Technol. 21(7), 593–597 (1998)
Lim, B.G., Ching, C.B., Tan, R.B.H.: Determination of competitive adsorption isotherms of enantiomers on dual-side adsorbent. Sep. Technol. 5, 213–228 (1995)
Maddox, I.S.: Use of silicalite for the adsorption of n-butanol from fermentation liquids. Biotechnol. Lett. 4, 759–760 (1982)
Morse, G., Jones, R., Thibault, J., Tezel, F.H.: Neural network modelling of adsorption isotherms. Adsorption 17, 303–309 (2011)
Nielsen, L., Larsson, M., Hoist, O., Mattiasson, B.: Adsorbents for extractive bioconversion applied to the acetone-butanol fermentation. Appl. Microbiol. Biotechnol. 28, 335–339 (1988)
Nielsen, D.R., Prather, K.J.: In situ product recovery of n-butanol using polymeric resins. Biotechnol. Bioeng. 102, 811–821 (2009)
Oudshoorn, A., Van der Wielen, L.A.M., Straathof, A.J.J.: Adsorption equilibria of bio-based butanol solutions using zeolite. Biochem. Eng. J. 48, 99–103 (2009)
Oudshoorn, A., Van derWielen, L.A.M., Straathof, A.J.J.: Desorption of butanol from zeolite material. Biochem. Eng. J. 67, 167–172 (2012)
Qureshi, N., Blaschek, H.P.: Production of acetone butanol ethanol (ABE) by a hyper-producing mutant strain of Clostridium beijerinckii BA101 and recovery by pervaporation. Biotechnol. Prog. 15, 594–602 (1999)
Qureshi, N., Hughes, S., Maddox, I.S., Cotta, M.A.: Energy-efficient recovery of butanol from model solutions and fermentation broth by adsorption. Bioprocess Biosyst. Eng. 27, 215–222 (2005)
Remi, J.C.S., Remy, T., Van Hunskerken, V., Van de Perre, S., Duerinck, T., Maes, M., De Vos, D., Gobechiya, E., Kirschock, C.E.A., Baron, G.V., Denayer, J.F.M.: Biobutanol separation with the metal-organic framework ZIF-8. ChemSusChem 4, 1074–1077 (2011)
Remi, J.C.S., Baron, G.V., Denayer, J.F.M.: Adsorptive separation for the recovery and purification of biobutanol. Adsorption 18, 367–373 (2012)
Ruthven, D.M.: Principles of adsorption and adsorption processes. Wiley, New York (1984)
Saravanan, V., Waijers, D.A., Ziari, M., Noordermeer, M.A.: Recovery of 1-butanol from aqueous solutions using zeolite ZSM-5 with a high Si/Al ratio; suitability of a column process for industrial applications. Biochem. Eng. J. 49, 33–39 (2010)
Shapovalov, O.I., Ashkinazi, L.A.: Biobutanol: biofuel of second generation. Russ. J. Appl. Chem. 81(12), 2232–2236 (2008)
Sharma, P., Chung, W.J.: Synthesis of MEL type zeolite with different kinds of morphology for the recovery of 1-butanol from aqueous solution. Desalination 275, 172–180 (2011)
Sowerby, B., Crittenden, B.D.: Vapour phase separation of alcohol water mixtures by adsorption onto silicalite. Gas Sep. Purif. 2, 177–183 (1988)
Thompson, A.B., Cope, S.J., Swift, T.D., Notestein, J.M.: Adsorption of n-butanol from dilute aqueous solution with grafted calixarenes. Langmuir 27, 11990–11998 (2011)
Wu, X.-H., Lin, B.-C.: Model modification of binary competitive isotherm. J. Liquid Chromatogr. Relat. Technol. 32, 2465–2483 (2009)
Wu, J., Zhuang, W., Ying, H.: Acetone-butanol-ethanol competitive sorption simulation from single, binary, and ternary systems in a fixed-bed of KA-I resin. Biotechnol. Prog. 31(1), 124–134 (2014)
Yang, M., Hubble, J., Fang, M., Locke, A.D., Rathbone, R.R.: A neural network for breakthrough prediction in packed bed adsorption. Biotechnol. Tech. 7(2), 155–158 (1993)
Yang, X., Tsai, G.J., Tsao, G.T.: Enhancement of in situ adsorption on the acetone-butanol fermentation by Clostridium acetobutylicum. Sep. Technol. 4, 81–92 (1994)
Zheng, Y.N., Li, L.Z., Xian, M., Ma, Y.J., Yang, J.M., Xu, X., He, D.Z.: Problems with the microbial production of butanol. J. Ind. Microbiol. Biotechnol. 36, 1127–1138 (2009)
Acknowledgments
The authors would like to acknowledge the Natural Science and Engineering Research Council (NSERC) of Canada and Ontario Graduate Scholarship (OGS) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdehagh, N., Tezel, F.H. & Thibault, J. Multicomponent adsorption modeling: isotherms for ABE model solutions using activated carbon F-400. Adsorption 22, 357–370 (2016). https://doi.org/10.1007/s10450-016-9784-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-016-9784-y