Abstract
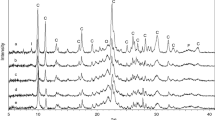
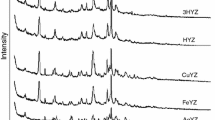
This study presents the results of the methane adsorption properties of clinoptilolite tuff from Bigadic, Turkey and that of acid treated forms at 273 and 293 K up to 100 kPa using volumetric apparatus. In order to assess changes in structural and gas adsorption properties of clinoptilolite, zeolite sample was treated with acid solutions of varying concentrations (0.1, 0.5, 1.0 and 2.0 M) at 70 °C during 3 h. Structural and thermal characterization of natural and acid treated clinoptilolite samples were carried out using a combination of techniques such as X-ray diffraction, X-ray fluorescence, thermogravimetric, differential thermal analysis and nitrogen adsorption methods. At both temperatures, uptake of methane (CH4) increased in the following order: CLN < CLN-H2 < CLN-H1 < CLN-H05 < CLN-H01. CH4 adsorption capacities of the original and acid treated clinoptilolites were found in the range of 0.476–0.910 mmol/g and 0.398–0.691 mmol/g at 273 and 293 K, respectively.





Similar content being viewed by others
Abbreviations
- CLN:
-
Original clinoptilolite
- CLN-H01:
-
Clinoptilolite treated with 0.1 M HCl solution
- CLN-H05:
-
Clinoptilolite treated with 0.5 M HCl solution
- CLN-H1:
-
Clinoptilolite treated with 1.0 M HCl solution
- CLN-H2:
-
Clinoptilolite treated with 2.0 M HCl solution
References
Ackley, M.W., Yang, R.T.: Adsorption characteristics of high-exchange clinoptilolites. Ind. Eng. Chem. Res. 30, 2523–2530 (1991)
Ackley, M.W., Giese, R.F., Yang, R.T.: Clinoptilolite: untapped potential for kinetic gas separations. Zeolites 12, 780–788 (1992)
Aguilar-Armenta, G., Hernandez-Ramirez, G., Flores-Loyola, E., Ugarte-Castaneda, A., Silva-Gonzalez, R., Tabares-Munoz, C., Jimenez-Lopez, A., Rodriguez-Castellon, E.: Adsorption kinetics of CO2, O2, N2, and CH4 in cation-exchanged clinoptilolite. J. Phys. Chem. B 105, 1313–1319 (2001)
Aguilar-Armenta, G., Patiño-Iglesias, M.E., Leyva-Ramos, R.: Adsorption kinetic behaviour of pure CO2, N2 and CH4 in natural clinoptilolite at different temperatures. Adsorpt. Sci. Technol. 21, 81–91 (2003)
Aguilar-Armenta, G., Romero-Perez, A.: Adsorption of C2H4, C2H6 and CO2 on cation-exchanged clinoptilolite. Adsorpt. Sci. Technol. 27, 523–536 (2009)
Ahmed, M.J., Theydan, S.K.: Equilibrium isotherms and adsorption heats analysis for ternary mixture of methane, ethane, and propane on 4A zeolite. J. Porous Mater. 21, 747–755 (2014)
Antoniou, M.K., Diamanti, E.K., Enotiadis, A., Policicchio, A., Dimos, K., Ciuchi, F., Maccallini, E., Gournis, D., Agostino, R.G.: Methane storage in zeolite-like carbon materials. Microporous Mesoporous Mater 188, 16–22 (2014)
Arcoya, A., Gonzalez, J.A., Travieso, N., Seoane, X.L.: Physicochemical and catalytic properties of a modified natural clinoptilolite. Clay Miner. 29(1), 123–131 (1994)
Arcoya, A., González, J.A., Llabre, G., Seoane, X.L., Travieso, N.: Role of the countercations on the molecular sieve properties of a clinoptilolite. Microporous Mater. 7(1), 1–13 (1996)
Barrer, R.M.: Zeolites and Clay Minerals as Sorbents and Molecular Sieves. Academic Press, New York (1978)
Berlier, K., Olivier, M.G., Jadot, R.: Adsorption of methane, ethane, and ethylene on zeolite. J. Chem. Eng. Data 40, 1206–1208 (1995)
Breck, D.W.: Zeolite Molecular Sieves. Wiley, New York (1984)
Brunauer, S., Deming, L.S., Deming, W.E., Teller, E.: On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 62, 1723–1732 (1940)
Cardarelli, F.: Materials Handbook: A Concise Desktop Reference, 2nd edn. Springer, New York (2008)
Carson, P., Mumford, C.: Hazardous Chemicals Handbook, 2nd edn. Butterworth-Heinemann, Burlington (2002)
Cavenati, S., Grande, C.A., Rodrigues, A.E.: Adsorption equilibrium of methane, carbon dioxide, and nitrogen on zeolite 13X at high pressures. J. Chem. Eng. Data 49, 1095–1101 (2004)
Christidis, G.E., Moraetis, D., Keheyan, E., Akhalbedashvili, L., Kekelidze, N., Gevorkyan, R., Yeritsyan, H., Sargsyan, H.: Chemical and thermal modification of natural HEU-type zeolitic materials from Armenia, Georgia and Greece. Appl. Clay Sci. 24(1–2), 79–91 (2003)
Choudhary, V.R., Mayadevi, S., Pal Singh, A.: Sorption isotherms of methane, ethane, ethene and carbon dioxide on NaX, NaY and Na-mordenite zeolites. J. Chem. Soc., Faraday Trans. 91, 2935–2944 (1995)
Çakıcıoğlu-Özkan, F., Ülkü, S.: The effect of HCl treatment on water vapor adsorption characteristics of clinoptilolite rich natural zeolite. Microporous Mesoporous Mater. 77, 47–53 (2005)
Ding, T.F., Ozawa, S., Yamazaki, T., Watanuki, I., Ogino, Y.: A generalized treatment of adsorption of methane onto various zeolites. Langmuir 4, 392–396 (1988)
Dyer, A.: An Introduction to Zeolite Molecular Sieves. Wiley, New York (1988)
Esenli, F., Sirkecioğlu, A.: The relationship between zeolite (heulandite–clinoptilolite) content and the ammonium-exchange capacity of pyrodastic rocks in Gordes, Turkey. Clay Miner. 40(4), 557–564 (2005)
Galli, E., Gottardi, G., Mayer, H., Preisinger, A., Passaglia, E.: The structure of potassium-exchanged heulandite at 293, 373 and 593 K. Acta Crystallogr. B 39, 189–197 (1983)
Garcia-Basabe, Y., Rodriguez-Iznaga, I., de Menorval, L., Llewellyn, P., Maurin, G., Lewisf, D.W., Binionsf, R., Autieg, M.A., Ruiz-Salvadora, R.: Step-wise dealumination of natural clinoptilolite: structural and physicochemical characterization. Microporous Mesoporous Mater. 135(1–3), 187–196 (2010)
Gottardi, G., Galli, E.: Natural Zeolites. Springer, Berlin (1985)
Grande, C.A., Blom, R.: Cryogenic adsorption of methane and carbon dioxide on zeolites 4A and 13X. Energy Fuels 28, 6688–6693 (2014)
Gregg, S.J., Sing, K.S.W.: Adsorption, Surface Area and Porosity, 2nd edn. Academic Press, London (1982)
Hamzah, S.A., Mahmood, N.Z., Sulaiman, A.H.: Ozone depletion substances (ODS) emission analysis from the life cycle of chemical substances and electricity used in potable water production in Malaysia. Aust. J. Basic & Appl. Sci. 4 (9), 4286–4293 (2010)
Hernandez-Beltran, N.A., Olguin, M.T.: Elemental composition variability of clinoptilolite-rich tuff after the treatment with acid phosphate solutions. Hydrometallurgy 89, 374–378 (2007)
Hernández-Huesca, R., Díaz, L., Aguilar-Armenta, G.: Adsorption equilibria and kinetics of CO2, CH4 and N2 in natural zeolites. Sep. Purif. Technol. 15, 163–173 (1999)
Jayaraman, A., Yang, R.T., Chinn, D., Munson, C.L.: Tailored clinoptilolites for nitrogen/methane separation. Ind. Eng. Chem. Res. 44, 5184–5192 (2005)
Korkuna, O., Leboda, R., Skubiszewska-Zeiba, J., Vrublevska, T., Gunko, V.M., Ryczkowski, J.: Structural and physicochemical properties of natural zeolites: clinoptilolite and mordenite. Microporous Mesoporous Mater. 87, 243–254 (2006)
Kouvelosa, E., Kesoreb, K., Steriotisa, T., Grigoropoulouc, H., Bouloubasid, D., Theophiloud, N., Tzintzosd, S., Kanelopoulosa, N.: High pressure N2/CH4 adsorption measurements in clinoptilolites. Microporous Mesoporous Mater. 99, 106–111 (2007)
Koyama, K., Takeuchi, Y.: Clinoptilolite: the distribution of potassium atoms and its role in thermal analysis. Z. Kristallogr. 145, 216–239 (1977)
Lide, D.R.: CRC Handbook of Chemistry and Physics. CRC Press, Boca Raton (2003)
Macedonia, M.D., Moore, D.D., Maginn, E.J., Olken, M.M.: Adsorption studies of methane, ethane, and argon in the zeolite mordenite: molecular simulations and experiments. Langmuir 16, 3823–3834 (2000)
Maple, M.J., Williams, C.D.: Separating nitrogen/methane on zeolite like molecular sieves. Microporous Mesoporous Mater. 111, 627–631 (2008)
Merkle, A.B., Slaughter, M.: Determination and refinement of the structure of heulandite. Am. Mineral. 53, 1120–1138 (1968)
Moore, D.M., Reynolds Jr, R.C.: X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd edn. Oxford University Press, New York (1997)
Mumpton, F.A.: Clinoptilolite redefined. Am. Mineral. 45, 351–369 (1960)
Patnaik, P.: Handbook of Environmental Analysis, Chemical Pollutants in Air, Water, Soil, and Solid Wastes, 2nd edn. CRC Press, Boca Raton (2010)
Petrakakis, Y., Mylona, E., Georgantas, D., Grigoropoulou, H.: Leaching of lead from clinoptilolite at acidic conditions. Global NEST J. 9(3), 207–213 (2007)
Predescu, L., Tezel, F.H., Stelmack, P.: Adsorption of Nitrogen and Methane on Natural Clinoptilolite. In: Bonneviot, L., Kaliaguin, S. (eds.) Zeolites: A Refined Tool for Designing Catalytic Sites, pp. 507–512. Elsevier, Amsterdam (1995)
Radosavljević-Mihajlović, A., Dondur, V., Daković, A., Lemić, J., Tomašević-Čanović, M.: Physicochemical and structural characteristics of HEU-type zeolitic tuff treated by hydrochloric acid. J. Serb. Chem. Soc. 69(4), 273–281 (2004)
Rozic, M., Cerjan-Stefanovic, S., Kurajica, S., Rozmari Maeefat, M., Margeta, K., Farkas, A.: Decationization and dealumination of clinoptilolite tuff and ammonium exchange on acid-modified tuff. J. Colloid Interface Sci. 284, 48–56 (2005)
Salvestrini, S., Sagliano, P., Iovino, P., Capasso, S., Colella, C.: Atrazine adsorption by acid activated zeolite-rich tuffs. Appl. Clay Sci. 49, 330–335 (2010)
Shindell, D.T., Faluvegi, G., Koch, D.M., Schmidt, G.A., Unger, N., Bauer, S.E.: Improved attribution of climate forcing to emissions. Science 326(5953), 716–718 (2009)
Triebe, R.W., Tezel, F.H., Khulbe, K.C.: Adsorption of methane, ethane and ethylene on molecular sieve zeolites. Gas Sep. Purif. 10, 81–84 (1996)
Tsitsishvili, G.V.: Physicochemical properties of high silica L and clinoptilolite zeolites. In: Meier, W.M., Uytterhoeven, J.B. (eds.) Molecular Sieves, pp. 291–298. ACS, Washington DC (1973)
Acknowledgments
Special thanks to Dr. Matthias Thommes for his helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alver, B.E., Sakizci, M. Influence of acid treatment on structure of clinoptilolite tuff and its adsorption of methane. Adsorption 21, 391–399 (2015). https://doi.org/10.1007/s10450-015-9679-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-015-9679-3