Abstract
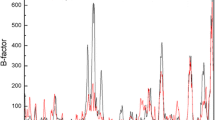
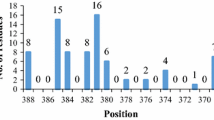
Quantum mechanical and molecular dynamics methods were used to analyze the structure and stability of neutral and zwitterionic configurations of the extracted active site sequence from a Burkholderia cepacia lipase, histidyl-seryl-glutamin (His86-Ser87-Gln88) and its mutated form, histidyl-cysteyl-glutamin (His86-Cys87-Gln88) in vacuum and different solvents. The effects of solvent dielectric constant, explicit and implicit water molecules and side chain mutation on the structure and stability of this sequence in both neutral and zwitterionic forms are represented. The quantum mechanics computations represent that the relative stability of zwitterionic and neutral configurations depends on the solvent structure and its dielectric constant. Therefore, in vacuum and the considered non-polar solvents, the neutral form of the interested sequences is more stable than the zwitterionic form, while their zwitterionic form is more stable than the neutral form in the aqueous solution and the investigated polar solvents in most cases. However, on the potential energy surfaces calculated, there is a barrier to proton transfer from the positively charged ammonium group to the negatively charged carboxylat group or from the ammonium group to the adjacent carbonyl oxygen and or from side chain oxygen and sulfur to negatively charged carboxylat group. Molecular dynamics simulations (MD) were also performed by using periodic boundary conditions for the zwitterionic configuration of the hydrated molecules in a box of water molecules. The obtained results demonstrated that the presence of explicit water molecules provides the more compact structures of the studied molecules. These simulations also indicated that side chain mutation and replacement of sulfur with oxygen leads to reduction of molecular flexibility and packing.

Similar content being viewed by others
References
Becke AD (1993) Density functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. doi:10.1063/1.464913
Berendsen HJC, Postma JPM, Van Gunsteren WF, Dinola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690. doi:10.1063/1.448118
Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M (1983) CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J Comp Chem 4:187–217. doi:10.1002/jcc.540040211
Brünger AT, Karplus M (1988) Polar hydrogen positions in proteins: Empirical energy placement and neutron diffraction comparison. Proteins Struct Funct Bioinformatics 4:148–156. doi:10.1002/prot.340040208
Creighton TE (1993) Proteins: structures and molecular properties. W.H. Freeman, New York
Faber K (1997) Biotransformations in organic chemistry, 3rd edn. Springer, Berlin
Foresman JB, Frisch Æ (1996) Exploring chemistry with electronic structure methods, 2nd edn. Gaussian Inc, Pittsburgh, PA
Frisch MJ, Trucks GW, Schlegel HB, Pople JA et al (1998) Gaussian. Inc, Pittsburgh, PA
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1988) NBO Version 3.1
Grochulski P, Li YG, Schrag JD, Bouthillier F, Smith P, Harrison D, Rubin B, Cygler M (1993) Insights into interfacial activation from an open structure of Candida rugosa lipase. J Mol Catal B: Enzym 268:12843–12847. doi:10.2210/pdb1crl/pdb
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723. doi:10.1002/elps.1150181505
Guieysse D, Salagnad C, Monsan P, Remaud-Simeon M, Tran V (2003) Towards a novel explanation of Pseudomonas cepacia lipase enantioselectivity via molecular modelling of the enantiomer trajectory into the active site. Tetrahedron Asym 14:1807–1817. doi:10.1016/S0957-4166(03)00374-4
Han D, Rhee JS (1986) Characteristics of lipase-catalyzed hydrolysis of olive oil in AOT-isooctane reversed micelles. Biotechnol Bioeng 28:1250–1255. doi:10.1002/bit.260280817
Havel HA (1996) Spectroscopic methods for determining protein structure in solution. VCH, New York
Honig B, Sharp K, Yang AS (1993) Macroscopic models of aqueous solutions: biological and chemical applications. J Phys Chem 97:1101–1109. doi:10.1021/j100108a002
Jacoby SLS, Kowalik KS, Pizzo JT (1972) Iterative methods for nonlinear optimization problems. Prentice-Hall, Englewood Cliffs, NJ
Jalkanen KJ, Suhai S (1996) N-acetyl-l-alanine N′-methylamide: a density functional analysis of the vibrational absorption and vibrational circular dichroism spectra. Chem Phys 208:81–116. doi:10.1016/0301-0104(96)00042-0
Jorgensen WL, Chandrasekhar J, Madura JD (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–936. doi:10.1063/1.445869
Kim KK, Song HK, Shin DH, Hwang KY, Suh SW (1997) The crystal structure of a triacylglycerol lipase from Pseudomonas cepacia reveals a highly open conformation in the absence of a bound inhibitor. Structure 5:173–185. doi:10.1016/S0969-2126(97)00177-9
Lang DA, Mannesse MLM, De Haas GH, Verheij HM, Dijkstra BW (1998) Structural basis of the chiral selectivity of Pseudomonas cepacia lipase. Eur J Biochem 254:333–340. doi:10.1046/j.1432-1327.1998.2540333.x
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. doi:10.1103/PhysRevB.37.785
Ljubovic E, Sunjic V (2000) Comparative study of conformational effects on stereoselective lipase catalysed acetylation of sec hydroxy groups in diastereomeric 14-membered lactones and their acyclic analogs. Tetrahedron Lett 41:9135–9138. doi:10.1016/S0040-4039(00)01634-8
Lui′c M, Tomi′c S, Leščić I, Ljubovi′c E, Sepac D, Sunji′c V, Vitale LJ, Saenger W, Koji′c-Prodi′c B (2001) Complex of Burkholderia cepacia lipase with transition state analogue of 1-phenoxy-2-acetoxybutane. Eur J Biochem 268:3964–3973. doi:10.1046/j.1432-1327.2001.02303.x
MacKerell ADJ, Bashford D, Bellott M et al (1998) All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B 102:3586–3616. doi:10.1021/jp973084f
Pugazhenthi G, Kumar A (2004) Enzyme membrane reactor for hydrolysis of olive oil using lipase immobilized on modified PMMA composite membrane. J Membr Sci 228:187–197. doi:10.1016/j.memsci.2003.10.007
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926. doi:10.1021/cr00088a005
Schmid RD, Verger R (1998) Lipases: interfacial enzymes with attractive applications. Angew Chem Int Ed Engl 37:1608–1633. doi:10.1002/(SICI)1521-3773(19980703)37:12<1608:AID-ANIE1608>3.0.CO; 2-V
Tafi A, van Almsick A, Corelli F, Crusco M, Laumen KE, Schneider MP, Botta M (2000) Computer simulations of enantioselective ester hydrolyses catalyzed by Pseudomonas cepacia lipase. J Org Chem 65:3659–3665. doi:10.1021/jo9919198
Tafi A, Manetti F, Botta M, Casati S, Santaniello E (2004) A drop of enantioselectivity in the Pseudomonas cepacia lipase-catalyzed ester hydrolysis is influenced by the chain length of the fatty acid. Tetrahedron Asym 15:2345–2350. doi:10.1016/j.tetasy.2004.06.010
Tajkhorshid E, Paizs B, Suhai S (1997) Conformational effects on the proton affinity of the Schiff base in bacteriorhodopsin: a density functional study. J Phys Chem B 101:8021–8028. doi:10.1021/jp971283t
Tomasi J, Persico M (1994) Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem Rev 94:2027–2094. doi:10.1002/chin.199508316
Tomić S, Kojić-Prodić B (2002) A quantitative model for predicting enzyme enantioselectivity: application to Burkholderia cepacia lipase and 3-(aryloxy)-1, 2-propanediol derivatives. J Mol Graph Model 21:241–252. doi:10.1016/S1093-3263(02)00148-1
Tuomi WV, Kazlauskas RJ (1999) Molecular basis for enantioselectivity of lipase from Pseudomonas cepacia toward primary alcohols. Modeling, kinetics, and chemical modification of Tyr29 to increase or decrease enantioselectivity. J Org Chem 64:2638–2647. doi: 10.1021/jo981783y
Wiberg KB (1965) A Scheme for strain energy minimization. Application to the cycloalkanes. J Am Chem Soc 87:1070–1078. doi:10.1021/ja01083a024
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tahan, A., Monajjemi, M. Solvent Dielectric Effect and Side Chain Mutation on the Structural Stability of Burkholderia cepacia Lipase Active Site: A Quantum Mechanical/Molecular Mechanics Study. Acta Biotheor 59, 291–312 (2011). https://doi.org/10.1007/s10441-011-9137-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10441-011-9137-x