Abstract
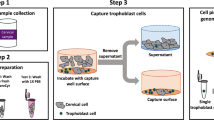
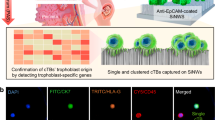
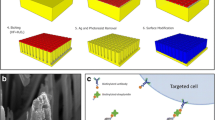
Placental trophoblast cells present in cervical samples have great potential towards non-invasive prenatal testing. However, cervical samples are highly heterogeneous, largely comprised of maternal cervical cells with only a small quantity of trophoblast cells. In order to use these rare cells for diagnostic applications, there is a need to enrich and isolate them from the heterogeneous maternal sample. Our goal was to investigate the use of gravitational flow on an inclined surface and optimize parameters including angle of incline, surface material, incubation time on the surface, solution volume, and device channel width in order to identify a design allowing label-free enrichment of trophoblast cells. In this work we detail the development of a new method and device for controlling cell adhesion to a surface vs. rolling into a collection area. The enrichment device design was developed for ease of use by non-specialized personal and on a slide surface for the ability to be directly integrated onto an automatic cell picker instrument, which can be used for downstream single cell isolation. JEG-3 trophoblast cells were used with clinical cervical samples to present the effect of the different optimization parameters on enrichment. We further provide an assessment of the impact shear stress and thickness of the liquid layer has on cell enrichment. We found that this method provides a maximum JEG-3 enrichment using polystyrene surfaces at a 50° incline with a 5 min incubation period prior to inclined flow. This resulted in a 396 ± 52% increase in purity of the trophoblast cells from the clinical cervical samples as confirmed using human leukocyte antigen G (HLA-G) antibody staining with fluorescence imaging to identify JEG-3 cells. Ultimately, this method is inexpensive, quick, and has the potential for direct integration into fetal cell isolation platforms.








Similar content being viewed by others
References
Akolekar, R., J. Beta, G. Picciarelli, C. Ogilvie, and F. D’Antonio. Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 45:16–26, 2015.
Bailey, C. M., A. Tripathi, and A. Shukla. Effects of flow and bulk vesicle concentration on supported lipid bilayer formation. Langmuir 33:11986–11997, 2017.
Bailey-Hytholt, C. M., T. Puranik, A. Tripathi, and A. Shukla. Investigating interactions of phthalate environmental toxicants with lipid structures. Colloids Surf. B: Biointerfaces 190:110923, 2020.
Bailey-Hytholt, C. M., S. Sayeed, M. Kraus, R. Joseph, A. Shukla, and A. Tripathi. A rapid method for label-free enrichment of rare trophoblast cells from cervical samples. Sci. Rep. 9:12115, 2019.
Bailey-Hytholt, C. M., T. L. Shen, B. Nie, A. Tripathi, and A. Shukla. Placental trophoblast-inspired lipid bilayers for cell-free investigation of molecular interactions. ACS Appl. Mater. Interfaces 12:31099–31111, 2020.
Ballard, P. L., and G. M. Tompkins. Glucocorticoid-induced alteration of the surface membrane of cultured hepatoma cells. J. Cell Biol. 47:222–234, 1970.
Benn, P., A. Borrell, R. W. K. Chiu, H. Cuckle, L. Dugoff, B. Faas, S. Gross, T. Huang, J. Johnson, R. Maymon, M. Norton, A. Odibo, P. Schielen, K. Spencer, D. Wright, and Y. Yaron. Position statement from the Chromosome Abnormality Screening Committee on behalf of the Board of the International Society for Prenatal Diagnosis. Prenat. Diagn. 35:725–734, 2015.
Benn, P., H. Cuckle, and E. Pergament. Non-invasive prenatal testing for aneuploidy: current status and future prospects. Ultrasound Obstet. Gynecol. 42:15–33, 2013.
Bolnick, A. D., R. Fritz, C. Jain, L. Kadam, J. M. Bolnick, B. A. Kilburn, M. Singh, M. P. Diamond, S. Drewlo, and D. R. Armant. Trophoblast retrieval and isolation from the cervix for noninvasive, first trimester, fetal gender determination in a carrier of congenital adrenal hyperplasia. Reprod. Sci. 23:717–722, 2016.
Bolnick, J. M., B. A. Kilburn, S. Bajpayee, N. Reddy, R. Jeelani, B. Crone, N. Simmerman, M. Singh, M. P. Diamond, and D. R. Armant. Trophoblast retrieval and isolation from the cervix (TRIC) for noninvasive prenatal screening at 5 to 20 weeks of gestation. Fertil. Steril. 102:135–142.e6, 2014.
Carlstedt, I., H. Lindgren, J. K. Sheehan, and U. Ulmstent. Isolation and characterization of human cervical-mucus glycoproteins. 211:13–22, 1983.
Chang, K. C., D. F. J. Tees, and D. A. Hammer. The state diagram for cell adhesion under flow: leukocyte rolling and firm adhesion. Proc. Natl. Acad Sci. 97(21):11262–11267, 2000.
Eniola, A. O., P. J. Willcox, and D. A. Hammer. Interplay between rolling and firm adhesion elucidated with a cell-free system engineered with two distinct receptor-ligand pairs. Biophys. J. 85:2720–2731, 2003.
Fagerberg, L., et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteomics 13:397–406, 2014.
Harris, L. K., C. J. P. Jones, and J. D. Aplin. Adhesion molecules in human trophoblast—a review II. Extravillous trophoblast. Placenta 30:299–304, 2009.
House, M., D. L. Kaplan, and S. Socrate. Relationships between mechanical properties and extracellular matrix constituents of the cervical stroma during pregnancy. Semin. Perinatol. 33:300–307, 2009.
Imudia, A. N., S. Kumar, M. P. Diamond, A. H. DeCherney, and D. R. Armant. Transcervical retrieval of fetal cells in the practice of modern medicine: a review of the current literature and future direction. Fertil. Steril. 93:1725–1730, 2010.
Imudia, A. N., Y. Suzuki, B. A. Kilburn, F. D. Yelian, M. P. Diamond, R. Romero, and D. R. Armant. Retrieval of trophoblast cells from the cervical canal for prediction of abnormal pregnancy: a pilot study. Hum. Reprod. 24:2086–2092, 2009.
Jain, C. V., L. Kadam, M. van Dijk, H.-R. Kohan-Ghadr, B. A. Kilburn, C. Hartman, V. Mazzorana, A. Visser, M. Hertz, A. D. Bolnick, R. Fritz, D. R. Armant, and S. Drewlo. Fetal genome profiling at 5 weeks of gestation after noninvasive isolation of trophoblast cells from the endocervical canal. Sci. Transl. Med. 8(363):363re4, 2016.
Ledingham, M. A., A. J. Thomson, F. Jordan, A. Young, M. Crawford, and J. E. Norman. Cell adhesion molecule expression in the cervix and myometrium during pregnancy and parturition. Obstet. Gynecol. 97:235–242, 2001.
Li, I. T. S., T. Ha, and Y. R. Chemla. Mapping cell surface adhesion by rotation tracking and adhesion footprinting. Sci. Rep. 7:1–11, 2017.
Liu, X., W. Gu, and X. Li. HLA-G regulates the invasive properties of JEG-3 choriocarcinoma cells by controlling STAT3 activation. Placenta 34:1044–1052, 2013.
Marshall, B. T., M. Long, J. W. Piper, T. Yago, R. P. McEver, and C. Zhu. Direct observation of catch bonds involving cell-adhesion molecules. Nature 423:190–193, 2003.
McEver, R. P., and C. Zhu. Rolling cell adhesion. Annu. Rev. Cell Dev. Biol. 26:363–396, 2010.
Moser, G., S. Drewlo, B. Huppertz, and D. R. Armant. Trophoblast retrieval and isolation from the cervix: origins of cervical trophoblasts and their potential value for risk assessment of ongoing pregnancies. Hum. Reprod. Update 24:484–496, 2018.
Olmsted, S. S., J. L. Padgett, A. I. Yudin, K. J. Whaley, T. R. Moench, and R. A. Cone. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys. J. 81:1930–1937, 2001.
Pfeifer, I., A. Benachi, A. Saker, J. P. Bonnefont, H. Mouawia, L. Broncy, R. Frydman, M. L. Brival, B. Lacour, R. Dachez, and P. Paterlini-Bréchot. Cervical trophoblasts for non-invasive single-cell genotyping and prenatal diagnosis. Placenta 37:56–60, 2016.
Pouyssegur, J., M. Willingham, and I. R. A. Pastan. Role of cell surface carbohydrates and proteins in cell behavior: studies on the biochemical reversion of an N-acetylglucosamine-deficient fibroblast mutant. Proc. Natl. Acad. Sci. 74:243–247, 1977.
Saha, A. K., P. Osmulski, S. F. Dallo, M. Gaczynska, T. H. M. Huang, and A. K. Ramasubramanian. Cholesterol regulates monocyte rolling through CD44 distribution. Biophys. J. 112:1481–1488, 2017.
Saltzman, W. M., L. Michael, K. J. Whaley, and R. A. Cone. Antibody diffusion in human cervical mucus. 66:508–515, 1994.
The Human Protein Atlas. The cervix-specific proteome.
Tilburgs, T., Â. C. Crespo, A. Van Der Zwan, B. Rybalov, T. Raj, B. Stranger, L. Gardner, A. Moffett, and J. L. Strominger. Human HLA-G+ extravillous trophoblasts: immune-activating cells that interact with decidual leukocytes. Proc. Natl. Acad. Sci. USA. 112:7219–7224, 2015.
Uhlen, M., et al. Tissue-based map of the human proteome. Science. 347:1260419–1260419, 2015.
Zhu, C., T. Yago, J. Lou, V. I. Zernitsyna, and R. P. McEver. Mechanisms for flow-enhanced cell adhesion. Ann Biomed Eng. 36:604–621, 2008.
Acknowledgments
The material is based upon work supported by the National Science Foundation Graduate Research Fellowship awarded to C.M.B.H., under Grant No. 1644760. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. We thank the Brown Design Workshop for the use of the laser cutter and Makerbot Replicator. We thank Perkin Elmer, Inc., including Morey Kraus and Richard Joseph, for prenatal testing discussions and cervical samples.
Conflict of interest
AT is a paid scientific advisor/consultant and lecturer for PerkinElmer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jennifer West oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Current address for Christina M. Bailey-Hytholt: Department of Chemical Engineering, Worcester Polytechnic Institute, Worcester, MA, 01609, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bailey-Hytholt, C.M., Sayeed, S., Shukla, A. et al. Enrichment of Placental Trophoblast Cells from Clinical Cervical Samples Using Differences in Surface Adhesion on an Inclined Plane. Ann Biomed Eng 49, 2214–2227 (2021). https://doi.org/10.1007/s10439-021-02742-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-021-02742-x