Abstract
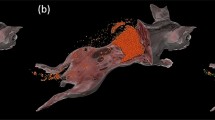
We demonstrated the use of multispectral cryo-imaging and software to analyze human mesenchymal stromal cells (hMSCs) biodistribution in mouse models of graft-versus-host-disease (GVHD) following allogeneic bone marrow transplantation (BMT). We injected quantum dot labeled MSCs via tail vein to mice receiving BMT and analyzed hMSC biodistribution in major organs (e.g. lung, liver, spleen, kidneys and bone marrow). We compared the biodistribution of hMSCs in mice following allogeneic BMT recipients (with GVHD) to the biodistribution following syngeneic BMT (without GVHD). Cryo-imaging system revealed cellular biodistribution and redistribution patterns in the animal model. We initially found clusters of cells in the lung that eventually dissociated to single cells and redistributed to other organs within 72 h. The in vivo half-life of the exogenous MSCs was about 21 h. We found that the biodistribution of stromal cells was not related to blood flow, rather cells preferentially homed to specific organs. In conclusion, cryo-imaging was suitable for analyzing the cellular biodistribution. It could provide capabilities of visualizing cells anywhere in the mouse model with single cell sensitivity. By characterizing the biodistribution and anatomical specificity of a therapeutic cellular product, we believe that cryo-imaging can play an important role in the advancement of stem and stromal cell therapies and regenerative medicine.







Similar content being viewed by others
References
Auletta, J. J., K. R. Cooke, L. A. Solchaga, R. J. Deans, and W. van’t Hof. Regenerative stromal cell therapy in allogeneic hematopoietic stem cell transplantation: current impact and future directions. Biol. Blood Marrow Transplant. 16:891–906, 2010.
Auletta, J. J., S. K. Eid, P. Wuttisarnwattana, I. Silva, L. Metheny, M. D. Keller, R. Guardia-Wolff, C. Liu, F. Wang, T. Bowen, Z. Lee, L. A. Solchaga, S. Ganguly, M. Tyler, D. L. Wilson, and K. R. Cooke. Human mesenchymal stromal cells attenuate graft-versus-host disease and maintain graft-versus-leukemia activity following experimental allogeneic bone marrow transplantation. Stem Cells 33:601–614, 2015.
Auletta, J. J., E. A. Zale, J. F. Welter, and L. A. Solchaga. Fibroblast growth factor-2 enhances expansion of human bone marrow-derived mesenchymal stromal cells without diminishing their immunosuppressive potential. Stem Cells Int. 2011:235176, 2011.
Barbash, I. M., P. Chouraqui, J. Baron, M. S. Feinberg, S. Etzion, A. Tessone, L. Miller, E. Guetta, D. Zipori, L. H. Kedes, R. A. Kloner, and J. Leor. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 108:863–868, 2003.
Barry, F. P., and J. M. Murphy. Mesenchymal stem cells: clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 36:568–584, 2004.
Bulte, J. W., I. D. Duncan, and J. A. Frank. In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J. Cereb. Blood Flow Metab. 22:899–907, 2002.
Chen, J., Y. Li, L. Wang, Z. Zhang, D. Lu, M. Lu, and M. Chopp. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32:1005–1011, 2001.
Copelan, E. A. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 354:1813–1826, 2006.
Dominici, M., K. Le Blanc, I. Mueller, I. Slaper-Cortenbach, F. Marini, D. Krause, R. Deans, A. Keating, D. Prockop, and E. Horwitz. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317, 2006.
Fischer, U. M., M. T. Harting, F. Jimenez, W. O. Monzon-Posadas, H. S. Xue, S. I. Savitz, G. A. Laine, and C. S. Cox. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 18:683–691, 2009.
Gao, X., L. W. Chung, and S. Nie. Quantum dots for in vivo molecular and cellular imaging. Methods Mol. Biol. 374:135–145, 2007.
Gao, J., J. E. Dennis, R. F. Muzic, M. Lundberg, and A. I. Caplan. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs 169:12–20, 2001.
Gargesha, M., M. Q. Qutaish, D. Roy, G. J. Steyer, M. Watanabe, and D. L. Wilson. Visualization of color anatomy and molecular fluorescence in whole-mouse cryo-imaging. Comput. Med. Imaging Graph. 35:195–205, 2011.
Li, H., X. Fu, Y. Ouyang, C. Cai, J. Wang, and T. Sun. Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res. 326:725–736, 2006.
Myers, J. T., D. S. Barkauskas, and A. Y. Huang. Dynamic imaging of marrow-resident granulocytes interacting with human mesenchymal stem cells upon systemic lipopolysaccharide challenge. Stem Cells Int. 2013:656839, 2013.
Najar, M., M. Krayem, M. Merimi, A. Burny, N. Meuleman, D. Bron, G. Raicevic, and L. Lagneaux. Insights into inflammatory priming of mesenchymal stromal cells: functional biological impacts. Inflamm. Res. 67:467–477, 2018.
Powell, K. A., and D. Wilson. 3-dimensional imaging modalities for phenotyping genetically engineered mice. Vet. Pathol. 49:106–115, 2012.
Qutaish, M. Q., K. E. Sullivant, S. M. Burden-Gulley, H. Lu, D. Roy, J. Wang, J. P. Basilion, S. M. Brady-Kalnay, and D. L. Wilson. Cryo-image analysis of tumor cell migration, invasion, and dispersal in a mouse xenograft model of human glioblastoma multiforme. Mol. Imaging Biol. 14:572–583, 2012.
Rabinovich, B. A., Y. Ye, T. Etto, J. Q. Chen, H. I. Levitsky, W. W. Overwijk, L. J. N. Cooper, J. Gelovani, and P. Hwu. Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proc. Natl. Acad. Sci. USA 105:14342–14346, 2008.
Rosen, A. B., D. J. Kelly, A. J. T. Schuldt, J. Lu, I. A. Potapova, S. V. Doronin, K. J. Robichaud, R. B. Robinson, M. R. Rosen, P. R. Brink, G. R. Gaudette, and I. S. Cohen. Finding fluorescent needles in the cardiac haystack: tracking human mesenchymal stem cells labeled with quantum dots for quantitative in vivo three-dimensional fluorescence analysis. Stem Cells 25:2128–2138, 2007.
Roy, D., M. Gargesha, G. J. Steyer, P. Hakimi, R. W. Hanson, and D. L. Wilson. Multi-scale characterization of the PEPCK-C mouse through 3D cryo-imaging. Int. J. Biomed. Imaging 2010:105984, 2010.
Roy, D., G. J. Steyer, M. Gargesha, M. E. Stone, and D. L. Wilson. 3D cryo-imaging: a very high-resolution view of the whole mouse. Anat. Rec. 292:342–351, 2009.
Schenk, S., N. Mal, A. Finan, M. Zhang, M. Kiedrowski, Z. Popovic, P. M. McCarthy, and M. S. Penn. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells 25:245–251, 2007.
Shaposhnikov, V. L. Distribution of bone-marrow cells in the mouse skeleton. Bull. Exp. Biol. Med. 87:510–512, 1979.
Steyer, G. J., F. Dong, L. Kanodia, D. Roy, M. Penn, and D. L. Wilson. Detection and quantification of fluorescent cell clusters in cryo-imaging. Int. J. Biomed. Imaging 2012:698413, 2012.
Steyer, G. J., D. Roy, O. Salvado, M. E. Stone, and D. L. Wilson. Removal of out-of-plane fluorescence for single cell visualization and quantification in cryo-imaging. Ann. Biomed. Eng. 37:1613–1628, 2009.
Walczak, P., D. A. Kedziorek, A. A. Gilad, B. P. Barnett, and J. W. Bulte. Applicability and limitations of MR tracking of neural stem cells with asymmetric cell division and rapid turnover: the case of the shiverer dysmyelinated mouse brain. Magn. Reson. Med. 58:261–269, 2007.
Weiss, A. R. R., and M. H. Dahlke. Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front. Immunol. 10:1191, 2019.
Wuttisarnwattana, P., M. Gargesha, W. van’t Hof, K. R. Cooke, and D. L. Wilson. Automatic stem cell detection in microscopic whole mouse cryo-imaging. IEEE Trans. Med. Imaging 35:819–829, 2016.
Acknowledgments
The research was supported by an Ohio Third Frontier Wright Projects Program award (OTFWPP) (D.L.W., co-I), National Center of Regenerative Medicine Pilot Grant (K.R.C.), the Ohio Board of Regents (K.R.C.), the Meredith Cowden Foundation (K.R.C.), The Thailand Research Fund MRG6080218 (P.W.), and the National Institute of Health through R42-CA124270, R41HD063241-01 (D.L.W.).
Conflict of interest
Dr. Gargesha (M.G.) declares that he is an employee of BioInVision Inc. which is a company that manufactures CryoViz™, a system that utilizes the cryo-imaging principle as the core technology. The rest of the authors (P.W., S.E., K.R.C., D.L.W.) declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Smadar Cohen oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wuttisarnwattana, P., Eid, S., Gargesha, M. et al. Cryo-imaging of Stem Cell Biodistribution in Mouse Model of Graft-Versus-Host-Disease. Ann Biomed Eng 48, 1702–1711 (2020). https://doi.org/10.1007/s10439-020-02487-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-020-02487-z