Abstract
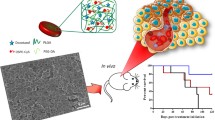
Targeting smaller populations of circulating tumor clusters (CTC) with tumor-initiating and colonization potentials at distant sites in circulation remains a challenge as clusters possess both epithelial and mesenchymal characteristics. Bullet shaped ellipsoidal nanostructures of size 600 ± 11.3 nm (major axis) and 281.9 ± 5.3 nm (minor axis) with 2.2 aspect ratio were self-assembled using inorganic and organic GRAS biomaterials to preferentially target tumor-causing CTCs. Negatively-charged chondroitin sulfate in presence of gelatin guides unidirectional growth of calcium carbonate mesocrystals to form nanobullets, mediates CD44 targeting of CTCs. Switchable multi-responsive drug release profiles (temperature and pH) were recorded for nanobullets promoting spontaneous and efficient cell-killing. CD44 and E-cadherin overexpressing ‘seeding’ cell clusters of 170 ± 22 µm were developed as in vitro CTC model. pH responsive release of Dox into lysosome stimulates calcium influx resulting in cell death. CD44-blocked CTCs showed significantly reduced internalization when compared to CD44-expressing CTCs thereby confirming CD44 specific internalization of nanobullets. Significantly retarded expansion of clusters when shifted to cell adhesive surfaces depicts the potential of nanobullets against colonization of CTCs. Hence, newer insights on developed anisotropic ECM-mimetic nanohybrids would enhance targeted capture of tumor-initiating clusters in systemic circulation that would potentially reduce the progression of tumor in breast cancer patients.








Similar content being viewed by others
References
Al-Hajj, M., M. S. Wicha, A. Benito-Hernandez, S. J. Morrison, and M. F. Clarke. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. 100:3983–3988, 2003.
Anurag Singh, J. S. EMT cancer stem cells and drug resistance. Oncogene 29:4741–4751, 2011.
Asimakopoulou, A. P., A. D. Theocharis, G. N. Tzanakakis, and N. K. Karamanos. The biological role of chondroitin sulfate in cancer and chondroitin-based anticancer agents. Vivo (Brooklyn) 22:385–390, 2008.
Bidard, F., C. Proudhon, and J. Pierga. Circulating tumor cells in breast cancer. Mol. Oncol. 10:418–430, 2016.
Chen, L., S. Xiao, H. Zhu, L. Wang, and H. Liang. Shape-dependent internalization kinetics of nanoparticles by membranes. Soft Matter 12:2632–2641, 2016.
Chen, C., S. Zhao, A. Karnad, and J. W. Freeman. The biology and role of CD44 in cancer progression: therapeutic implications. J. Hematol. Oncol. 11:1–23, 2018.
Dasgupta, A., A. R. Lim, and C. M. Ghajar. Circulating and disseminated tumor cells: harbingers or initiators of metastasis? Mol. Oncol. 11:40–61, 2017.
De Lima, J. M., R. R. Sarmento, J. R. De Souza, F. A. Brayner, A. Paula, S. Feitosa, R. Padilha, L. C. Alves, I. J. Porto, R. Ferreti, B. Dantas, J. E. De Oliveira, E. S. De Medeiros, P. Rogério, F. Bonan, and L. R. Castellano. Evaluation of hemagglutination activity of chitosan nanoparticles using human erythrocytes. Biomed Res. Int. 3–9:2015, 2015.
Dhandapani, R., S. Sethuraman, and A. Subramanian. Nanohybrids—cancer theranostics for tiny tumor clusters. J. Control. Release 299:21–30, 2019.
Dong, Z., L. Feng, W. Zhu, X. Sun, M. Gao, H. Zhao, Y. Chao, and Z. Liu. CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials 110:60–70, 2016.
Eroglu, Z., O. Fielder, and G. Somlo. Analysis of circulating tumor cells in breast cancer. J. Natl. Compr. Cancer Netw. 11:977–985, 2013.
Fu, W., M. H. Mohd Noor, L. M. Yusof, T. A. T. Ibrahim, Y. S. Keong, A. Z. Jaji, and M. Z. A. B. Zakaria. In vitro evaluation of a novel pH sensitive drug delivery system based cockle shell-derived aragonite nanoparticles against osteosarcoma. J. Exp. Nanosci. 8080:1–22, 2017.
Giuliano, M., A. Shaikh, H. C. Lo, G. Arpino, S. De Placido, X. H. Zhang, M. Cristofanilli, R. Schiff, and M. V. Trivedi. Perspective on circulating tumor cell clusters: why it takes a village to metastasize. Cancer Res. 78:845–852, 2018.
Hong, Y., F. Fang, and Q. Zhang. Circulating tumor cell clusters: what we know and what we expect. Int. J. Oncol. 49:2206–2216, 2016.
Jiang, H., X. Y. Liu, G. Zhang, and Y. Li. Kinetics and template nucleation of self-assembled hydroxyapatite nanocrystallites by chondroitin sulfate. J. Biol. Chem. 280:42061–42066, 2005.
Liu, Z., Y. Xiao, W. Chen, Y. Wang, B. Wang, and G. Wang. Calcium phosphate nanoparticles primarily induce cell necrosis through lysosomal rupture: the origination of material cytotoxicity. J. Mater. Chem. B 2:3480–3489, 2014.
Liu, L., X. Zhang, X. Liu, J. Liu, G. Lu, D. L. Kaplan, H. Zhu, and Q. Lu. Biomineralization of stable and monodisperse vaterite microspheres using silk nanoparticles. ACS Appl. Mater. Interfaces 7:1735–1745, 2015.
Man, Y., Q. Wang, and W. Kemmner. Currently used markers for CTC isolation—advantages, limitations and impact on cancer prognosis. J. Clin. Exp. Pathol. 01:1–7, 2011.
Micalizzi, D. S., S. Maheswaran, and D. A. Haber. A conduit to metastasis: circulating tumor. Cell Biol. 31:1827–1840, 2017.
Monzavi-Karbassi, B., J. S. Stanley, L. Hennings, F. Jousheghany, C. Artaud, S. Shaaf, and T. Kieber-Emmons. Chondroitin sulfate glycosaminoglycans as major P-selectin ligands on metastatic breast cancer cell lines. Int. J. Cancer 120:1179–1191, 2007.
Nagarajan, S., L. Soussan, M. Bechelany, C. Teyssier, V. Cavaillès, C. Pochat-Bohatier, P. Miele, N. Kalkura, J. M. Janot, and S. Balme. Novel biocompatible electrospun gelatin fiber mats with antibiotic drug delivery properties. J. Mater. Chem. B 4:1134–1141, 2016.
Poruk, K. E., A. L. Blackford, M. J. Weiss, J. L. Cameron, J. He, M. Goggins, Z. A. Rasheed, C. L. Wolfgang, and L. D. Wood. Circulating tumor cells expressing markers of tumor-initiating cells predict poor survival and cancer recurrence in patients with pancreatic ductal adenocarcinoma. Clin. Cancer Res. 23:2681–2690, 2017.
Punnoose, E. A., S. K. Atwal, J. M. Spoerke, H. Savage, A. Pandita, A. Pirzkall, B. M. Fine, L. C. Amler, D. S. Chen, and M. R. Lackner. Molecular biomarker analyses using circulating tumor cells. PLoS ONE 5:e1257, 2010.
Siegel, R. L., K. D. Miller, and A. Jemal. Cancer statistics, 2018. CA. Cancer J. Clin. 68:7–30, 2018.
Soundararajan, A., J. Muralidhar, R. Dhandapani, J. Radhakrishnan, A. Manigandan, S. Kalyanasundaram, S. Sethuraman, and A. Subramanian. Surface topography of polylactic acid nanofibrous mats: influence on blood compatibility. J. Mater. Sci. Mater. Med. 29:145, 2018.
Spaeth, E. L., A. M. Labaff, B. P. Toole, A. Klopp, M. Andreeff, and F. C. Marini. Mesenchymal CD44 expression contributes to the acquisition of an activated fibroblast phenotype via TWIST activation in the tumor microenvironment. Cancer Res. 73:5347–5359, 2013.
Thapa, R., and G. D. Wilson. The importance of CD44 as a stem cell biomarker and therapeutic target in cancer. Stem Cells Int. 2016:2087204, 2016.
Yang, X., S. K. Sarvestani, S. Moeinzadeh, X. He, and E. Jabbari. Effect of CD44 binding peptide conjugated to an engineered inert matrix on maintenance of breast cancer stem cells and tumorsphere formation. PLoS ONE 8:1–15, 2013.
Yu, M., A. Bardia, B. S. Wittner, S. L. Stott, M. E. Smas, D. T. Ting, S. J. Isakoff, J. C. Ciciliano, M. N. Wells, A. M. Shah, K. F. Concannon, M. C. Donaldson, L. V. Sequist, E. Brachtel, D. Sgroi, J. Baselga, S. Ramaswamy, and M. Toner. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339(80):580–584, 2013.
Zhang, Y., Y. Lv, Y. Niu, H. Su, and A. Feng. Role of circulating tumor cell (CTC) monitoring in evaluating prognosis of triple-negative breast cancer patients in China. Med. Sci. Monit. 23:3071–3079, 2017.
Acknowledgments
Authors thank PG-Teaching (SR/NM/PG-04/2015), Nano Mission (SR/NM/NS-1205/2015(G), FIST (SR/FST/LSI-327/2007, SR/FST/LSI-622/2014), Department of Science and Technology, Government of India for financial support. First Author is thankful to Council of Scientific and Industrial Research for senior research fellowship (09/1095(0022)/18-EMR-I), Government of India.
Conflict of interest
Authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Konstantinos Konstantopoulos oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dhandapani, R., Subramanian, A. & Sethuraman, S. ECM-Mimetic Multiresponsive Nanobullets Targeted Against Metastasizing Circulating Tumor Clusters in Breast Cancer. Ann Biomed Eng 48, 568–581 (2020). https://doi.org/10.1007/s10439-019-02370-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02370-6