Abstract
Aim
This study aimed to explore the knowledge, attitude, and practice that community pharmacists of Lebanon hold with regard to checking for drug interactions.
Subjects and methods
This cross-sectional study involved data collected from 89 anonymous and self-administered survey questionnaires by community pharmacists from community pharmacies dispersed amongst the six governorates of Lebanon. It also entailed individual interviews with a self-selected few. Knowledge, attitude, and practice with regard to drug interactions were collected.
Results
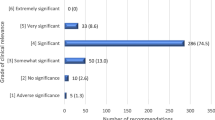
There is a large gap in the knowledge of the community pharmacists about drug interactions with other drugs, food, and herbs. When searching for interactions, pharmacists were found to refer mostly to the internet, drug applications, or colleagues. Their attitudes were positive and coherent with understanding their role in clarifying interactions when prescribing drugs; nevertheless, their practices were suboptimal.
Conclusion
Community pharmacists around Lebanon are aware of their role and responsibility concerning drug interactions though only a few do so in their daily work. Suggested interventions include further training and workshops to refresh the memories of these pharmacists on different interactions, and implementing software programs in pharmacies to detect these interactions on the spot.



Similar content being viewed by others
References
Alshakka MA, Ibrahim MI, Hassali MA (2013) Do health professionals have positive perception towards consumer reporting of adverse drug reactions? J Clin Diagn Res 7:2181–2185
Bates DW, CullenDJ LN, Petersen LA, Small SD, Servi D et al (1995) Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA 274:29–34
Boyce RD, Ragueneau-Majlessi I, Yu J, Tay-Sontheimer J, Kinsella C, Chou E et al (2018) Developing user personas to aid in the design of a user-centered natural product-drug interaction information resource for researchers. AMIA Annu Symp Proc 2018:279–287
FDA (2019) Drug development and drug interactions. https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions [Accessed Jan 2019]
Figueiras A, Herdeiro MT, Polonia J, Gestal-Otero JJ (2006) An educational intervention to improve physician reporting of adverse drug reactions: a cluster-randomized controlled trial. JAMA 296:1086–1093
Glassman PA, Simon B, Belperio P, Lanto A (2002) Improving recognition of drug interactions: benefits and barriers to using automated drug alerts. Med Care 40:1161–1171
Hajj A, Hallit S, Ramia E, Salameh P (2018) Medication safety knowledge, attitudes and practices among community pharmacists in Lebanon. Curr Med Res Opin 34:149–156
Hajjar R, Bassatne A, Cheaito MA, El Naser DR, Traboulsy S, Haddadin F et al (2017) Characterizing the interaction between physicians, pharmacists and pharmaceutical representatives in a middle-income country: a qualitative study. PLoS One 12:e0184662
Indermitte J, Erba L, Beutler M, Bruppacher R, Haefeli WE, Hersberger KE (2007) Management of potential drug interactions in community pharmacies: a questionnaire-based survey in Switzerland. Eur J Clin Pharmacol 63:297–305
Irujo M, Beitia G, Bes-Rastrollo M, Figueiras A, Hernandez-Diaz S, Lasheras B (2007) Factors that influence under-reporting of suspected adverse drug reactions among community pharmacists in a Spanish region. Drug Saf 30:1073–1082
Iskandar K, Hallit S, Raad EB, Droubi F, Layoun N, Salameh P (2017) Community pharmacy in Lebanon: a societal perspective. Pharm Pract (Granada) 15:893
Ismail MY (2009) Drug food interactions and role of pharmacist. Asian J Pharm Clin Res 2(4):1–10
Ko Y, Malone DC, Skrepnek GH, Armstrong EP, Murphy JE, Abarca J et al (2008) Prescribers' knowledge of and sources of information for potential drug–drug interactions: a postal survey of US prescribers. Drug Saf 31:525–536
MacKeigan LD, Dolovich L, Petrovic B, MacCallum L, Bojarski EA, Pojskic N (2018) Audit of community pharmacists' prescribing interventions: quality assessment of a newly reimbursed service. J Am Pharm Assoc (2003) 58:622–629
Mallet L, Spinewine A, Huang A (2007) The challenge of managing drug interactions in elderly people. Lancet 370:185–191
Pronsky ZM, Crowe SJP (2016) Clinical food–drug interactions. In: Mahan LK, Escott-Stump S, Raymond J (Eds.) Krause's food & the nutrition care process,13th edition. Elsevier/Saunders, St Louis, MO, pp 209–228
Ramia E, Zeenny RM, Hallit S, Salameh P (2017) Assessment of patients' knowledge and practices regarding their medication use and risks in Lebanon. Int J Clin Pharm 39:1084–1094
Shah A (2009) Pharmacy intervention in the medication use process: the role of pharmacists in improving patient safety. https://www.fip.org/files/content/priority-areas/patient-safety/patientsafetyadvidshah.pdf [Accessed Jan 2019]
Sinha HK (2014) Role of pharmacists in retailing of drugs. J Adv Pharm Technol Res 5:107
Suriyapakorn B, Chairat P, Boonyoprakarn S, Rojanarattanangkul P, Pisetcheep W, Hunsakunachai N et al (2019) Comparison of potential drug–drug interactions with metabolic syndrome medications detected by two databases. PLoS One 14:e0225239
UK General Medical Council (2013) Good practice in prescribing and managing medicines and devices. https://www.gmc-uk.org/-/media/documents/Prescribing_guidance.pdf_59055247.pdf [Accessed Jan 2019]
van Grootheest K, Olsson S, Couper M, de Jong-van den Berg L (2004) Pharmacists' role in reporting adverse drug reactions in an international perspective. Pharmacoepidemiol Drug Saf 13:457–464
Vohra S, Cvijovic K, Boon H, Foster BC, Jaeger W, LeGatt D et al (2012) Study of natural health product adverse reactions (SONAR): active surveillance of adverse events following concurrent natural health product and prescription drug use in community pharmacies. PLoS One 7:e45196
WHO (2002) Safety of medicines: A guide to detecting and reporting adverse drug reactions. WHO, Geneva. http://archives.who.int/tbs/safety/esd_safety.pdf [Accessed Jan 2019]
WHO (2017). Patient safety: making health care safer. WHO, Geneva. https://apps.who.int/iris/bitstream/handle/10665/255507/WHO-HIS-SDS-2017.11-eng.pdf?sequence=1&isAllowed=y [Accessed Jan 2019]
Yu YM, Lee E, Koo BS, Jeong KH, Choi KH, Kang LK et al (2016) Predictive factors of spontaneous reporting of adverse drug reactions among community pharmacists. PLoS One 11:e0155517
Zeenny R, Wakim S, Kuyumjian YM (2017) Potentially inappropriate medications use in community-based aged patients: a cross-sectional study using 2012 Beers criteria. Clin Interv Aging 12:65–73
Acknowledgements
The authors would like to thank the participants for their time and contribution, Dr. Pascale Salameh from the Lebanese Order of Pharmacists for her expert advice, and Nour Naji and Mona Moukaddem for their assistance in survey collection.
The authors would also like to thank the American University of Beirut Faculties of Health Sciences and Medicine for the collaborative “research methods” course to second-year medical students from which this project emanated.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by all authors. Data analysis was performed by Nour Makkaoui, Zahraa Atoui, and Nathalie K. Zgheib. The first draft of the manuscript was written by Nour Makkawi, Adham Halaoui, and Zahraa Atoui, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study was reviewed and approved by the Institutional Review Board (IRB) of the American University of Beirut under the following number: Pharmaco.NZ.25/SBS-2017-0549. All participants were informed about the purpose of the study and the option to withdraw at any time. They all orally consented to take part of the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 32 kb)
Rights and permissions
About this article
Cite this article
Makkaoui, N., Halaoui, A., Atoui, Z. et al. Knowledge, attitudes, and practices regarding drug interactions among community pharmacists. J Public Health (Berl.) 29, 1357–1363 (2021). https://doi.org/10.1007/s10389-020-01252-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10389-020-01252-9