Abstract
Purpose
To report aging-associated change rates in circumpapillary retinal nerve fiber layer thickness (cpRNFLT) and macular ganglion cell-inner plexiform layer and complex thickness (MGCIPLT, MGCCT) in normal Japanese eyes and to compare the data in linear scaled visual field (VF) sensitivity of central 4 points of Humphrey Field Analyzer (HFA) 24-2 test (VF4TestPoints) to that in MGCIPLT in four 0.6-mm-diameter circles corresponding to the four central points of HFA 24-2 adjusted for retinal ganglion cell displacement (GCIPLT4TestPoints).
Study design
Prospective observational study
Methods
HFA 24-2 tests and spectral-domain optical coherence tomography (SD-OCT) measurements of cpRNFLT, MGCIPLT, MGCCT and GCIPLT4TestPoints were performed every 3 months for 3 years in 73 eyes of 37 healthy Japanese with mean age of 50.4 years. The time changes of SD-OCT-measured parameters and VF4TestPoints were analyzed using a linear mixed model.
Results
The aging-associated change rates were -0.064 μm/year for MGCIPLT and and -0.095 for MGCCT (P=0.020 and 0.017), but could not be detected for cpRNFLT. They accelerated with aging at -0.009μm/year/year of age for MGCIPLT (P<0.001), at 0.011 for MGCCT (P<0.001) and at 0.013 for cpRNFLT(0.031). The aging-associated decline of -82.1 [1/Lambert]/year of VF4TestPoints corresponded to -0.095 μm/year of GCIPLT4TestPoints.
Conclusion
We report that aging-associated change rates of cpRNFLT, MGCIPLT and MGCCT in normal Japanese eyes were found to be significantly accelerated along with aging. Relationship between VF sensitivity decline rates and SD-OCT measured GCIPLT decline rates during physiological aging in the corresponding parafoveal retinal areas are also documented.
Similar content being viewed by others
Introduction
Histologic studies in human eyes have estimated that about 7000 retinal ganglion cells (RGCs) are lost during normal aging [1,2,3,4,5]. In accordance with these findings, cross-sectional and longitudinal studies in normal human eyes show that visual field (VF) sensitivity measured using standard automated perimetry (SAP) [6,7,8,9,10] and the thickness of the RGC-related retinal layers (RGC-RRLT) determined by spectral-domain optical coherence tomography (SD-OCT) showed physiological aging-associated decline [11,12,13,14,15,16,17,18,19,20,21,22]. To estimate disease-caused time-related changes of RGC-RRLT, it is mandatory to deduct the physiological aging-associated changes from the measured values.
It is well known that there are ethnical differences in the RGC-RRLT measurement results in normal subjects [14, 15, 21], and that RGC-RRLTs measurement results strongly depend on the SD-OCT instruments used [23,24,25,26,27]. Although there are reports of aging-associated decline rates of RGC-RRLT in other ethnical populations [17,18,19,20,21,22], there is a paucity of information on the SD-OCT instrument-specific aging-associated decline rates of RGC-RRLT in healthy Japanese. Both glaucoma and the physiological aging process reduce the RGC-RRLT and VF sensitivity [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. In glaucoma eyes, the decline rates of SD-OCT measured RGC-RRLT and VF sensitivity in the corresponding retinal area were compared, and in glaucoma eyes with early or pre-perimetric damage, changes in the circumpapillary retinal nerve fiber layer thickness (cpRNFLT) are reported to generally precede glaucomatous declines in the VF sensitivity detected using the Humphrey Field Analyzer 24-2 Swedish Interactive Thresholding Algorithm (SITA) program (HFA 24-2, Carl Zeiss Meditec) [28,29,30,31]. However, a comparison of aging-associated declines in SD-OCT-measured RGC-RRLT and VF sensitivity in the corresponding retinal areas are not reported in the same subjects’ eyes.
The purpose of the current study was two-fold. 1) To prospectively measure the longitudinal time changes in the cpRNFLT, macular ganglion cell-inner plexiform layer thickness (MGCIPLT) and ganglion cell complex thickness (MGCCT) and report the aging-associated decline rate of cpRNFLT, MGCIPLT and MGCCT in normal Japanese, and 2) to compare the aging-associated changes of the MGCIPLT to VF sensitivity obtained in the corresponding retinal area in the same eye.
Methods
Subjects. Self-reported healthy Japanese individuals were recruited at the Tajimi Eye Clinic (Tajimi, Gifu, Japan). After subjects were screened verbally and medical histories recorded, an ocular examination was performed that included measurements of the uncorrected and autorefraction-corrected visual acuity (VA) with a Landolt chart at 5 meters and the corneal curvature using an autorefractometer (KR-800A, Topcon). In addition, the central corneal thickness and axial length (AXL) were measured, respectively, using a specular microscope (SP-3000P, Topcon) and the IOLMaster (Carl Zeiss Meditec). The VFs were examined using SAP (HFA 24-2 SITA program, Carl Zeiss Meditec). The VF examination was repeated whenever it was considered unreliable or outside the normal limits. The SD-OCT examination was followed by dilated optic disc stereo photography and fundus photography, dilated funduscopy, slit-lamp biomicroscopy, and intraocular pressure (IOP) measurements by Goldmann applanation tonometry. A pair of sequential stereoscopic optic nerve head photographs at a parallax of about 8 degrees (30-degree angle of view) and non-stereoscopic fundus photographs (45-degree angle of view) was obtained using a digital fundus camera (TRC-NW7, Topcon) after pupillary dilation with 1.0% tropicamide. All ocular examinations were performed bilaterally.
The inclusion criteria were age between 20 and 75 years; normal eye examinations without any clinically significant cataract, ocular media, vitreoretinal, or choroidal abnormalities; IOP of 21 mmHg or lower; best-corrected decimal VA of 1.0 or higher; spherical refraction of ± 6 diopters (D) or less; astigmatism of 2 D or less; AXL of 26 mm or less; no previous ocular surgery; normal VF test results with the glaucoma hemifield test, and mean deviation and pattern standard deviation within normal limits. Subjects were excluded if the VF results were unreliable based on the perimetrist’s notes and reliability indices with fixation loss and false positive rates of over 20% and over 15%, respectively; the optic disc stereo photographs were of insufficient quality; or the OCT images were of insufficient quality (typically truncated B-scans and scans with a manufacturer-authorized image quality score of 30 or lower). After enrollment, routine ophthalmic examinations, SD-OCT, and VF measurements were prospectively performed every 3 months for 3 years.
The Review Board and Ethics Committee of Gifu Prefecture Medical Association approved the study (reference number, 25-1-001), which adhered to the tenets of the Declaration of Helsinki. The study was registered in the University Hospital Medical Information Network Clinical Trial Registry (UMIN-000012412). Each subject provided written informed consent after receiving a full explanation of the study protocol.
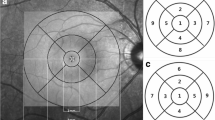
SD-OCT. SD-OCT data sets were obtained using a 3D-OCT 2000 (Topcon) with the horizontal 3-dimensional (3D) scan protocol in which data were obtained from 6.0 × 6.0-mm-square areas (512 A-scans × 128 frames) centered on the disc and with the vertical 3D scan protocol in which data were obtained from a 7.0 × 7.0-mm-square area (512 A-scans × 128 frames) centered on the fovea over a period of about 1.5 seconds for each scan. The points on the OCT image sensor plane corresponding to the measurement targets were determined according to the manufacturer-provided calculations; these calculated the relationship between the points in the posterior fundus of each subject eye and those on the SD-OCT image obtained from each eye based on refractive error, corneal radius, AXL of each subject eye and Gullstrand schematic eye [32] (Supplement). The data obtained in the presence of eye movements were discarded and the examination was repeated. Images also were excluded whenever they were affected by involuntary blinking or saccades, indicated by breaks, shifting of the vessels, or the presence of a straight line across the fundus OCT image, or had an image quality score of 30 or less. OCT measurements were repeated 3 times within several-second intervals, and the image with the best quality was used. The disc barycenter was determined using the raster-scan data, and the fovea identified in the OCT image as the thinnest pixel between the inner limiting membrane and the photoreceptor inner/outer segment junction (ellipsoid zone) adjacent to the fixation point. The RNFL and GCIPL (GCC) were segmented automatically in all B-scan images [33]. To minimize variability due to misplacement of the measurement location and/or segmentation error, an experienced researcher (T.K.) checked the locations of the disc barycenter and fovea and all layer segmentation in all images. The thickness of RNFL was measured along the 3.4-mm-diameter circle centered on the disc center (cpRNFLT), and that of macular GCIPL (GCC) {MGCIPLT(MGCCT)} was obtained from a 6.0 × 6.0-mm-square area centered on the fovea.
The MGCIPLT in a 0.6-mm-diameter circular retinal area (corresponding to about 2 degrees of visual angle) corresponding to each of the four central test points of the HFA 24-2 and adjusted for the RGC displacement [34] was obtained and the mean of these 4 measurement results were obtained (GCIPLT4TestPoints). The diameter of the retinal area (2 degrees) was like the grid size of the HFA 10-2 test program and roughly twice as large as the size-III stimulus point movements during fixation, such as drift.
Data analysis
Since no previous studies report the aging-associated change rates of cpRNFLT, MGCCT or MGCIPLT in normal Japanese eyes, the variation of the physiological aging-associated change rates of these SD-OCT parameters in Japanese needed for sample size calculation was unknown. When the study was planned, four papers already reported the physiological aging-related changes of cpRNFLT and MGCCT or MGCIPLT in normal subjects using the cohorts mainly consisting of Caucasians [17,18,19,20]. In these studies, the mean number of normal subjects included was 35, follow-up period was 36.3 months, follow-up interval was 4.4 months and there were 8 visits during the follow-up. Based on these studies, we planned to enroll 38 normal subjects who were to be examined at 3 month-intervals for 36 months (12 visits) and assumed that, in that way reliable data of physiological aging-associated changes of CpRNFLT, MGCCT or MGCIPLT in normal Japanese could be collected.
The results are expressed as the mean (standard deviation). The effects of aging (duration or time lapse from the baseline measurement) on the cpRNFLT, MGCIPLT, MGCCT and GCIPLT4TestPoints were analyzed, using the time changes of the cpRNFLT, MGCIPLT, MGCCT and GCIPLT4TestPoints values during follow-up as dependent variables and using the linear mixed model, which considers correlations between the paired eyes and measurement values from the same eyes. The explanatory variables were duration (time lapse from the baseline measurement), baseline age, baseline cpRNFLT, MGCIPLT, MGCCT or GCIPLT4TestPoints, AXL [12, 16] and gender [35,36,37], image quality index [38, 39].
The decibel values for VF sensitivity in test points of the HFA 24-2 test program were anti-logged to obtain the sensitivity in the linear scale (1/Lambert=10(0.1×dB), linear sensitivity) [40, 41]. Effects of aging (duration or time lapse from the baseline measurement) on the mean linear VF sensitivity over the whole area of the HFA-24-2 (VFmean) and the central 4 test points of the HFA 24-2 (VF4TestPoints) were analyzed, using the time changes of the VFmean or VF4TestPoints values during follow-up as a dependent variable and a linear mixed model, which considers correlations between the paired eyes and measurement values from the same eyes. The explanatory variables were duration (time lapse from the baseline measurement), baseline age, baseline VFmean and VF4TestPoint and AXL [10, 42, 43].
STATA software (version 17.0, Stata Corp) and The JMP® Pro 13 software (SAS Institute Inc.) were used for analyses and contribution of an explanatory variable with P<0.050 was adopted as to be significant.
Results
A total of 76 eyes of 38 normal subjects were enrolled; one eye of one subject was excluded because of development of epiretinal membrane and vitreoretinal traction during follow-up, and two eyes of one subject were excluded because reliable SD-OCT measurements could not always be obtained mainly due to saccades and blinking. The demographics of the remaining 73 eyes of 37 subjects are shown in Table 1. During the 3-year prospective follow-up, ocular transparent media including lens showed no changes on biomicroscopic examination.
Repeatability of cpRNFLT, MGCIPLT, MGCCT and GCIPLT4TestPoints measurement results calculated using the two measurements obtained at enrollment during two separate sessions [44] were 3.1, 1.1, 1.9 and 1.6 μm, respectively. Aging-associated change rates of cpRNFLT were not significantly different from zero (coefficient for duration or time lapsed was not significantly different from zero), but higher subjects’ baseline age showed significant negative correlation to aging associated change rates of cpRNFLT {-0.013 µm/year/year of age (P=0.031) in an eye with average parametric values of the current cohort (Table 1)} (interaction between duration and baseline age was significant at P=0.029). Baseline thickness, AXL or gender showed no significant effects on the aging associated change rates of cpRNFLT (P > 0.292) (Table 2). On the other hand, both MGCIPLT and MGCCT significantly declined with aging (P=0.020 and 0.017, respectively) and their aging-associated declining rates in an eye with average parametric values of the current cohort (Table 1) were − 0.064 and 0.095 µm/year, respectively. Further, aging-associated decline rates of MGCIPLT and MGCCT were greater along with their baseline thickness, subject’s baseline age and AXL {interaction between duration and baseline thickness, subject’s baseline age or AXL with duration was significant (P < 0.001 – 0.027)} (Tables 3 and 4). Aging-associated changes of the GCIPLT4TestPoints of an eye with average parametric values of this cohort (Table 1) showed similar tendency to that of the MGCIPLT, i.e., the GCIPLT over the whole macular area. Aging-associated decline rates of GCIPLT4TestPoints were not significant (− 0.095 µm/year, P=0.066), but the effect of subjects baseline age and AXL on the aging-associated decline rates of GCIPLT4TestPoints was significant, being close to the value obtained for MGCIPLT (− 0.011 µm/year/year of age, P=0.003, and − 0.098µm/year/mm, P=0.026 versus − 0.009 µm/year/year of age, P < 0.001, and − 0.061µm/year/year of age, P=0.011, respectively) (Table 5).
During the 3-year prospective follow-up, ocular transparent media including lens showed no changes on biomicroscopic examination. The mean sensitivity over the whole field (VFmean) and that of the four central test points of the HFA 24-2 (VF4TestPoints) of an eye with the average parametric values of this cohort (Table 1) showed significantly negative longitudinal time changes (physiological aging-associated declines) of − 35.1 and − 82.1[1/Lambert]/year (P<0.001) (corresponding to − 0.15 and − 0.19 dB/year), respectively, significantly more negative by − 2.1 and − 4.0 [1/Lambert]/years with older baseline ages (P < 0.001), and by − 0.10 and − 0.14 [1/Lambert]/[1/Lambert] with higher baseline sensitivities (P < 0.001), respectively. Further, the VFmean and VF4TestPoints measurement results themselves were also significantly lower by − 5.4 and − 13.8 [l/Lambert]/year with older baseline age (P < 0.001), and higher by 0.64 and 0.47 [1/Lambert]/[1/Lambert] with higher baseline sensitivity (P < 0.001), respectively (Tables 6 and 7).
Discussion
In the current study, the longitudinal aging-associated changes in the SD-OCT-measured thicknesses of the RGC-related retinal layers (RGC-RRLT) were studied in normal Japanese subjects with an average age of 50 years. Because of well-known ethnic differences in the RGC-RRLT measurement results in normal subjects [14, 15, 21], and dependence of the measurement results on the SD-OCT instruments used [23,24,25,26,27], it is of primary importance to provide aging-associated longitudinal change rates of cpRNFLT, MGCIPLT or MGCCT in healthy eyes for each ethnicity and record the SD-OCT instrument used to correctly estimate glaucoma-caused longitudinal changes of these important parameters.
Using Cirrus HD-OCT (Carl Zeiss Meditec), aging associated decline rates from − 0.16 to − 0.52 μm/year and of − 0.32 μm/year are reported for cpRNFLT [17, 20, 22] and for MGCIPLT [18], respectively, in normal subjects of mainly European descent. Using Spectralis OCT (Heidelberg Engineering GmbH), aging associated decline rates of − 0.44μm/year are reported for cpRNFLT in persons of European descent [19, 21] and of − 0.51μm/year in persons of African descent [21] all of them healthy subjects. In the current study where aging-associated change rates of cpRNFLT, MGCIPLT and MGCCT were measured using 3D-OCT 2000 (Topcon) every 3 months for 3 years in 37 normal Japanese subjects (mean age, 50.4 years), For cpRNFLT, aging-associated decline could not be detected, while MGCIPLT and MGCCT showed significant aging-associated decline of − 0.064 and − 0.095 μm/year, respectively. The findings obtained for cpRNFLT were unexpected, since VF sensitivity should approximately correspond to the retinal area covered by cpRNFLT in the same eye; the mean sensitivity over the HFA 24-2 VF, VFmean, showed significant aging-associated decline rate of − 35.1[1/Lambert)]/year, corresponding to − 0.15 dB/year which agreed with that reported by a cross-sectional and longitudinal study in normal Japanese [10]. This reasonable result obtained for VF sensitivity in the same eyes suggests that the current subjects were not significantly biased from the general normal population.
Larger test-retest variability, fewer measurement points, and a shorter follow-up period should reduce the power to detect a significant trend. Inter-visit test-retest variabilities of the cpRNFLT, MGCIPLT, MGCCT and GCIPLT4TestPoints with the current instruments were 3.1 μm, for cpRNFLT and 1.1 μm, 1.9 μm and 1.6 μm for MGCIPLT, MGCCT and GCIPLT4TestPoints, respectively, favorably compared with those reported in the literature for measurement results with various SD-OCT instruments [44, 45]. The follow-up period and intervals in the current study were similar to (3 years vs. 1.7–4.5 years and 3 months vs. 3.5–6 months, respectively) those adopted by previous studies [17,18,19,20]. Thus, it seems unlikely that a shorter follow-up period or fewer measurements mainly accounted for an undetected significant trend in longitudinal decline in the cpRNFLT in the current subjects. The current study indicates that in older subjects, the aging-associated decline rates in the cpRNFLT were found to be significantly more negative (interaction between the subject’s baseline age and duration were significant) by about − 0.01 μm/year/year of age, suggesting that in the cohort older than the current ones with mean age of 50.4 years, the aging-associated decline rates of the cpRNFLT might become sufficiently negative to be detected. The fact that the same analysis, when applied to the older group of the current subjects aged 50.4 years or older did not yield a significantly negative aging-associated decline rates does not necessarily contradict with the above speculation, since this stratified analysis included only 39 eyes of 20 subjects, hence half the number of subject eyes and the same number of explanatory variables should have considerably reduced the statistical power of detection (Supplementary Table 1). Somewhat younger ages of subjects in the current study than in the previous studies (50 vs. 56–65 years) [17,18,19,20,21,22] may be at least partly responsible for the discrepancy between the current and their results. Rates of aging-associated declines in the cpRNFLT were reportedly less than predicted by aging-associated decline in the number of RGCs, attributed to the presence of differential aging-associated declines of the non-neuronal components in the RNFL [46, 47]. An ethnic difference in the cpRNFLT has been reported [14, 15] and a comparison of the Bruch membrane’s opening-minimumu rim width and cpRNFLT between normal Japanese and Caucasians subjects suggests a difference in the ratio of the amount of RGC axons to that of the non-neuronal components in the RNFL between them [48]. Provided there are ethnic differences in the amount of physiological aging-associated changes in the non-neuronal component between Japanese subjects and other groups, this might partly explain the fact that aging-associated cpRNFLT decline could be detected in European subjects [17,18,19,20,21,22], but not in the current Japanese subjects. On the other hand, aging-associated decline rates of MGCIPLT and MGCCT were significant averaging − 0.064 μm and − 0.095μm/year, respectively. However, these rates also seemed to be considerably smaller than − 0.32 μm/year reported in normal subjects of mainly European descent [18], suggesting possibility that there is an ethnic difference in aging-associated decline rates of RGC-RRLTs and rates were smaller in Japanese than in European descent subjects. The fact that a small decline rate could be detected for MGCIPLT or MGCCT, but not for cpRNFLT, could be also compatible with the lower measurement repeatability of cpRNFLT than that of MGCIPLT or MGCCT (3.1 μm vs 1.1 μm or 1.9 μm). For cpRNFLT, MGCIPLT and MGCCT, aging-associated change rates were found to be significantly more negative along with thicker baseline thickness and higher subjects’ baseline age. Further, for MGCIPLT and MGCCT, rates were significantly more negative along with longer AXL or higher myopic power. Positive correlation of decline rates with thicker baseline thickness is understandable, given that a same aging-associated loss rate of each RGC, a greater number of baseline RGCs (∝ thicker baseline cpRNFLT, MGCIPLT or MGCCT) will result in a greater number of RGCs yearly lost (∝ yearly decline rate of cpRNFLT, MGCIPLT or MGCCT). Positive correlation of subjects’ age and AXL with MGCIPLT and/or MGCCT decline rate is interesting, but understandable, since both subjects’ age and myopia [49, 50] are well known risk factors for development of open angle glaucoma.
In the current study, the longitudinal aging-associated changes in the VF sensitivity and SD-OCT-measured RGC-RRLT in the corresponding area were measured in the same eyes of normal Japanese subjects. The correspondence should be more exact between the mean sensitivity of the central 4 test points of HFA 24-2, VF4TestPoints and GCIPLT4TestPoints than between the mean sensitivity over the HFA 24-2 VF, VFmean, and cpRNFLT. So, it may be interesting to compare the corresponding VF4TestPoints and GCIPLT4TestPoints. Although GCIPLT4TestPoints decline rate was not statistically significant (P=0.066), it should be greater than of MGCIPLT of -0.064 μm/year which was statistically significant, since GCIPLT4TestPoints was thicker than MGCIPLT and decline rates of MGCIPLT were significantly greater as baseline thickness increased (interaction between baseline thickness and duration was significant). Further, similar effects of baseline age and AXL on the thickness decline rate were seen for both MGCIPLT and GCIPLT4TestPoints. So, GCIPLT4TestPoints decline rate of − 0.095 μm/year is thought to be not far from the reality. The decline rate of VF linear sensitivity of -82.1[1/Lambert]/year (− 0.19 dB/year) in VF4TestPoints corresponded to that of − 0.095 μm/year in GCIPLT4TestPoints.
Using cross-sectional data of normal Japanese with the mean age of 49 years, we previously estimated that aging associated decline rate inVF4TestPoints of 1000/[1/Lambert]/decade roughly corresponds to that of a decline in GCIPLT4TestPoints of 1.6 μm/decade [16]. If, based on the current results of a yearly decline rate in VF4TestPoints and GCIPLT4TestPoints, we estimate the yearly decline rate of GCIPLT4TestPoints against a yearly decline rate of VF4TestPoints of 1000[1/Lambert]/decade, calculated to be 1.2 μm/decade, there will be a reasonable agreement with the value of 1.6 μm/decade, provided we take the difference in the cross-sectional and longitudinal analysis results into consideration.
Not only aging-associated changes in the retinal neurons but also in the neurons in the central visual pathway are related to a physiologic aging-associated decline in the perceived VF sensitivity in a specific retinal area. Since the cpRNFLT is related to the number of RGC axons and non-neuronal cells in the peripapillary retina, but not to neurons in the central visual pathway, the physiologic aging processes at age 50 years might be reflected with more sensitivity in the perceived VF sensitivity decline than in the OCT-measured cpRNFLT, which may partly be attributable to the fact that a significant physiological aging-associated decline in the perceived VF sensitivity is associated with insignificant physiological aging-associated decline in the OCT-measured cpRNFLT revealed by the present study. It is possible that the relationship between the OCT-measured structure and SAP-measured function (VF sensitivity) is not the same between the physiological aging process [16] and glaucoma-caused damaging process [51]. Glaucomatous damage primarily affects the RGCs and their axons and secondarily the neurons in the central visual pathway. In this case, the perceived SAP sensitivity decline may have been dampened by the plasticity of the visual cortex and normal cerebral adaptation to chronic deterioration in the visual input caused by slowly progressing glaucomatous RGC damage [52].
The current results have clinical implications in managing Japanese glaucoma patients using the SD-OCT 2000 (Topcon), which is widely used in Japan. To evaluate glaucoma-caused structural deterioration rates of MGCIPLT or MGCCT in patients with mean age of 50 years, aging-associated decline rates of MGCIPLT and MGCCT of − 0.064 and − 0.095 μm/year must be discounted. Further, for correct inter-group comparisons of cpRNFLT, MGCIPLT or MGCCT, adjustments must be made not only for subjects’ age, but also for AXL must be adjusted for correct inter-group comparison of MGCIPLT and MGCCT. A small time-dependent change of cpRNFLT might not be sensitively detected using SD-OCT 2000 in normal Japanese eyes aged between 40 and 60 years. If it could be detected, the detected change might be mainly attributable to a disease.
The current study has limitations. First, the number of normal subjects was small and the follow-up period may not have been sufficiently long, resulting in a lower statistical capability to detect significant time changes in the RGC-related retinal layer thickness measured using a current SD-OCT instrument. As discussed previously, however, the cpRNFLT or GCIPLT time change rates of about 0.3–0.5 μm/year are reported in previous studies with a similar number of normal subjects and follow-up periods [17,18,19,20,21], and the test-retest variation of the current SD-OCT 2000 measurement results was thought to be reasonably satisfactory compared with those reported for other SD-OCT instruments [44, 45]. These results suggest that the statistical power to detect the time change rates of the CpRNFLT in the current normal subjects should be comparable to those of previous studies that detected significant aging-related declines of the cpRNFLT [17,18,19,20,21]. The mean age of the current normal Japanese subjects was 50 years and, as discussed previously, the physiologic aging-related decline of the RGC-RRLTs would be greater in normal Japanese subjects with a higher age. Thus, it should be noted that the current results were applicable to Japanese subjects aged around 50 years. Finally, the current results were obtained using Topcon SD-OCT 2000 in normal Japanese. Since there exist reported ethnic differences in the SD-OCT-measured RGC-related retinal layers [14, 15, 21] and the thickness measurement results obtained with different SD-OCT instruments, these are not necessarily interchangeable [23,24,25,26,27], it must be noted that the current results can be used as a reference point to those obtained with Topcon SD-OCT in Japanese.
References
Balazsi AG, Rootman J, Drance SM, Schulzer M, Douglas GR. The effect of age on the nerve fiber population of the human optic nerve. Am J Ophthalmol. 1984;97:760–6.
Repka MX, Quigley HA. The effect of age on normal human optic nerve fiber number and diameter. Ophthalmology. 1989;96:26–32.
Mikelberg FS, Drance SM, Schulzer M, Yidegiligne H, Weis MM. The normal human optic nerve. Axon count and axon diameter distribution. Ophthalmology. 1989;96:1125–8.
Jonas JB, Schmidt AM, Müller-Bergh JA, Shlotzer-Schrehardt UM, Naumann GO. Human optic nerve fiber count and optic disc size. Invest Ophthalmol Vis Sci. 1992;33:2012–8.
Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41:741–8.
Heijl A, Lindgren G, Olsson J. Normal variability of static perimetric threshold values across the central visual field. Arch Ophthalmol. 1978;105:1544–9.
Lachenmayr BJ, Kojetinsky S, Ostermaier N, Angstwurm K, Vivell PMO, Schaumberger M. The different effects of aging on normal sensitivity in flicker and light-sense perimetry. Invest Ophthalmol Vis Sci. 1994;35:2741–8.
Hermann A, Paetzold J, Vonthein R, Krapp E, Rauscher S, Schiefer U. Age-dependent normative values for differential luminance sensitivity in automated static perimetry using the Octopus 101. Acta Ophthalmol. 2008;86:446–55.
Spry PGD, Johnson CA. Changes of the normal visual field: an age-old problem. Optom Vis Sci. 2001;78:436–41.
Iwase A, Fujii M, Murata H, Ohno Y, Araie M. Effects of physiologic myopia and aging on visual fields in normal eyes. Am J Ophthalmol. 2021;230:224–33.
Hirasawa H, Tomidokoro A, Araie M, Konno S, Saito H, Iwase A, et al. Peripapillary retinal nerve fiber layer thickness determined by spectral-domain optical coherence tomography in ophthalmologically normal eyes. Arch Ophthalmol. 2010;128:1420–6.
Mwanza JC, Durbin MK, Budenz DL, Girkin CA, Leung CK, Liebmann JM, Cirrus OCT Normative Database Study Group, et al. Profile and predictors of normal ganglion cell-inner plexiform layer thickness measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:7872–9.
Ooto S, Hangai M, Tomidokoro A, Saito H, Araie M, Otani T, et al. Effects of age, sex, and axial length on the three-dimensional profile of normal macular layer structures. Invest Ophthalmol Vis Sci. 2011;52:8769–79.
Girkin CA, McGwin G Jr, Sinai MJ, Sekhar GC, Fingeret M, Wollstein G, et al. Variation in optic nerve and macular structure with age and race with spectral-domain optical coherence tomography. Ophthalmology. 2011;118:2403–8.
Knight OJ, Girkin CA, Budenz DL, Durbin MK, Feuer WJ, Cirrus OCT Normative Database Study Group. Effect of race, age, and axial length on optic nerve head parameters and retinal nerve fiber layer thickness measured by Cirrus HD-OCT. Arch Ophthalmol. 2012;130:312–8.
Araie M, Saito H, Tomidokoro A, Murata H, Iwase A. Relationship between macular inner retinal layer thickness and corresponding retinal sensitivity in normal eyes. Invest Ophthalmol Vis Sci. 2014;55:7199–205.
Leung CKS, Yu M, Weinreb RN, Ye C, Liu S, Lai G, et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography. A prospective analysis of age-related loss. Ophthalmology. 2012;119:731–7.
Leung CKS, Ye C, Weinreb RN, Yu M, Lai G, Lam DS. Impact of age-related change of retinal nerve fiber layer and macular thicknesses on evaluation of glaucoma progression. Ophthalmology. 2013;120:2485–92.
Vianna JR, Danthurebandara VM, Sharpe GP, Hutchison DM, Belliveau AC, Shuba LM, et al. Importance of normal aging in estimating the rate of glaucomatous neuroretinal rim and retinal nerve fiber layer loss. Ophthalmology. 2015;122:2392–8.
Hammel L, Belghith A, Weinreb RN, Medeiros FA, Mendoza N, Zangwill LM. Comparing the rates of retinal nerve fiber layer and ganglion-cell-inner plexiform layer loss in healthy eyes and in glaucomatous eyes. Am J Ophthalmol. 2017;178:38–50.
Bowd C, Zangwill LM, Weinreb RB, Girkin CA, Fazio MA, Liebmann JM, et al. Racial differences in rate of change of spectral domain OCT-measured minimum rim width and retinal nerve fiber thickness. Am J Ophthalmol. 2018;196:154–64.
Öhnell HM, Heijl A, Bengtsson B. Ageing and glaucoma progression on the retinal nerve fibre layer using spectral-domain optical coherence tomography analysis. Acta Ophthalmol. 2021;99:260–8.
Pierro L, Giatsidis SM, Mantovani E, Gagliardi M. Macular thickness interoperator and intraoperator reproducibility in healthy eyes using 7 optical coherence tomography instruments. Am J Ophthalmol. 2010;150:199–204.
Leite MT, Rao HL, Weinreb RN, Zangwill LM, Bowd C, Sample PA, et al. Agreement among spectral-domain optical coherence tomography instruments for assessing retinal nerve fiber layer thickness. Am J Ophthalmol. 2011;151:85–92.
Kanamori A, Nakamura M, Tomioka M, Kawaka Y, Yamada Y, Negi A. Agreement Among three types of spectral-domain optical coherent tomography instruments in measuring parapapillary retinal nerve fibre layer thickness. Br J Ophthalmol. 2012;96:832–7.
Pierro L, Gagliardi M, Iuliano L, Ambrosi A, Bandello F. Retinal nerve fiber layer thickness reproducibility using seven different OCT instruments. Invest Ophthalmol Vis Sci. 2012;53:5912–20.
Mahmoudinezhad G, Mohammadzadeh V, Amini N, Delao K, Zhou B, Hong T, et al. Detection of longitudinal ganglion cell/inner plexiform layer change: comparison of two spectral-domain optical coherence tomography devices. Am J Ophtalmol. 2021;231:1–10.
Lisboa R, Leite MT, Zangwill LM, Tafreshi A, Weinreb RN, Medeiors FA. Diagnosing preperimetric glaucoma with spectral domain optical coherencetomography. Ophthalmology. 2012;119:2261–9.
Leung CK, Yu M, Weinreb RN, Lai G, Xu G, Lam DS. Retinal nerve fiber layer with spectral-domain optical coherence tomography: patterns of retinal nerve fiber layer progression. Ophthalmology. 2012;119:1858–66.
Kuang TM, Zhang C, Zangwill LM, Weinreb RN, Medeiros FA. Estimating lead time gained by optical coherence tomography in detecting glaucoma before development of visual field defects. Ophthalmology. 2015;122:2002–9.
Liu T, Tatham AJ, Gracitelli CP, Zangwill LM, Weinreb RN, Medeiros FA. Rates of retinal nerve fiber layer loss in contralateral eyes of glaucoma patients with unilateral progression by conventional methods. Ophthalmology. 2015;122:2243–51.
Iwase A, Sekine A, Suehiro J, Tanaka K, Kawasaki Y, Kawasaki R, et al. A new method of magnification correction for accurately measuring retinal vessel calibers from fundus photographs. Invest Ophthalmol Vis Sci. 2017;58:1858–64.
Yang Q, Reisman CA, Wang Z, Fukuma Y, Hangai M, Yoshimura N, et al. Automated layer segmentation of macular OCT images using dual-scale gradient information. Opt Express. 2010;18:21293–307.
Drasdo N, Millican CL, Katholi CR, Curcio CA. The length of Henle fibers in the human retina and a model of ganglion receptive field density in the visual field. Vis Res. 2007;47:2901–11.
Khawaja AP, Chan MP, Garway-Heath DF, Broadway DC, Luben R, Sherwin JC, et al. Associations with retinal nerve fiber layer measurements in the EPIC-Norfolk Eye Study. Invest Ophthalmol Vis Sci. 2013;54:5028–34.
Li D, Rauscher FG, Choi EY, Wang M, Baniasadi N, Wirkner K, et al. Sex-specific differences in circumpapillary retinal nerve fiber layer thickness. Ophthalmology. 2020;127:357–68.
Song WK, Lee SC, Lee ES, Kim CY, Kim SS. Macular thickness variations with sex, age, and axial length in healthy subjects: a spectral domain-optical coherence tomography study. Invest Ophthalmol Vis Sci. 2010;51:3913–8.
Russel DJ, Fallah S, Lier CF, Riffenburgh RH. A comprehensive model for correcting RNFL readings of varying signal strengths in Cirrus optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:7297–302.
Xu X, Xiao H, Lai K, Guo X, Luo J, Liu X. Determinants of macular ganglion cell-inner plexiform layer thickness in normal Chinese adults. BMC Ophthalmol. 2021;21:267.
Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26:688–710.
Harwerth RS, Wheat JI, Fredette MJ, Anderson DR. Linking structure and function in glaucoma. Prog Retin Eye Res. 2010;29:249–71.
Aung T, Foster PJ, Seah SK, Chan SP, Lim WK, Wu HM, et al. Automated static perimetry: the influence of myopia and its method of correction. Ophthalmology. 2001;108:290–5.
Tay E, Seah SK, Chan SP, Lim AT, Chew SJ, Foster PJ, et al. Optic disk ovality as an index of tilt and its relationship to myopia and perimetry. Am J Ophthalmol. 2005;139:247–52.
Araie M. Test-retest variability in structural parameters measured with glaucoma imaging devices. Jpn J Ophthalmol. 2013;57:1–24.
Mwanza JC, Oakley JD, Budenz DL, Chang RT, Knight OJ, Feuer WJ. Macular ganglion cell-inner plexiform layer: automated detection and thickness reproducibility with spectral domain-optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci. 2011;52:8323–9.
Harwerth RS, Wheat JL, Rangaswamy NV. Age-related loss of retinal ganglion cells and axons. Invest Ophthalmol Vis Sci. 2008;49:4437–43.
Patel NB, Lim M, Gajjar A, Evans KB, Harwerth RS. Age-associated changes in the retinal nerve fiber layer and optic nerve head. Invest Ophthalmol Vis Sci. 2014;55:5134–43.
Araie M, Iwase A, Sugiyama K, Nakazawa T, Tomita G, Hangai M, et al. Determinants and characteristics of Bruch’s membrane opening and Bruch’s membrane opening-minimum rim width in a normal Japanese population. Invest Ophthalmol Vis Sci. 2017;58:4106–13.
Ernest PJ, Schouten JS, Beckers HJ, Hendrikse F, Prins MH, Webers CA. An evidence-based review of prognostic factors for glaucomatous visual field progression. Ophthalmology. 2013;120:512–9.
Marcus MW, de Vries MM, Junoy Montolio FG, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011;118:1989–94.
Araie M, Murata H, Iwase A, Hangai M, Sugiyama K, Yoshimura N. Differences in relationship between macular inner retinal layer thickness and retinal sensitivity in eyes with early and progressed glaucoma. Invest Ophthalmol Vis Sci. 2016;57:1588–94.
Safran AB, Landis T. From cortical plasticity and unawareness of visual field defects. J Neuroophthalmol. 1999;19:84–8.
Acknowledgements
Santen Pharmaceuticals and Topcon Co. Ltd provided a portion of the research funding, but these funding had no role in the conduct of this research result.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
A. Iwase, Consulting fees (Santen), Payment or honoraria for lectures (ZEISS, Crewt Medical Systems, Heidelberg Engineering, Santen, Senju, Otsuka, Novartis), Patents planned, issued or pending (Topcon without any royalties) ; T. Higashide, Payment or honoraria for lectures (Santen, Senju, Novartis, Bayer, Kowa, Otsuka, Nitto, Viatris), Payment or honoraria for educational events (HOYA); M. Araie, Consulting fees (Aerie, Santen, Senju, Kowa), Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Santen), Patents planned, issued or pending (Topcon without any royalties), Participation on a Data Safety Monitoring Board or Advisory Board (Topcon); M. Fujii, None; Y. Ohno, None; Y. Tanaka, Employee (Santen); T. Kikawa, Employee (Topcon).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Aiko Iwase
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Iwase, A., Higashide, T., Fujii, M. et al. Aging-associated changes of optical coherence tomography-measured ganglion cell-related retinal layer thickness and visual sensitivity in normal Japanese. Jpn J Ophthalmol 68, 117–125 (2024). https://doi.org/10.1007/s10384-024-01049-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-024-01049-3