Abstract
Purpose
To determine the spatial distribution types of macular pigment (MP) in elderly Japanese individuals and to consider their origin.
Study design
Observational case series.
Methods
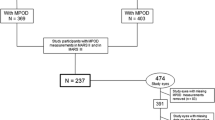
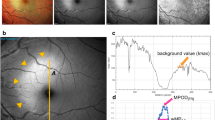
Local MP optical density (MPOD) at some eccentricities and MP volume were measured using the MPOD module of a MultiColor Spectralis in 96 pseudophakic eyes of 96 participants (age range, 52–86 years; mean age, 72.8 ± 8.3 years). The MP distribution types were determined from the MP spatial profiles. The retinal thickness (RT) at the foveal center, at both 0.5° and 0.9° eccentricities, and the foveal width were measured using optical coherence tomography.
Results
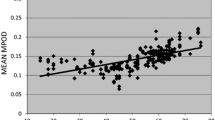
The mean local MPOD at the foveal center was 0.79. Spatial distribution was classified into four types: central peak (24.0%), ring-like (40.6%), intermediate (22.9%), and central dip (12.5%). The ring-like type was the most frequent in these Japanese participants. The central-peak type showed lower MPOD than did the other types in the area outside 0.9°. The ring-like type occurred frequently in eyes with small RT at 0.5° and wider foveal width. A rough contour of the Müller cell cone was found more frequently in the central-dip type than in the other types.
Conclusions
The present characteristics of the different distribution patterns could be explained by the hypothesis that MP presents mainly in the Müller cell cone within 0.5° and in Müller cells in the outer and inner plexiform layers in the area outside 0.5°. The anatomic characteristics of Müller cells at the fovea and parafovea likely affect the MP distribution.







Similar content being viewed by others
References
Bone RA, Landrum JT, Hime GW, Cains A, Zamor J. Stereochemistry of the human macular carotenoids. Invest Ophthalmol Vis Sci. 1993;34:2033–40.
Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 2001;385:28–40.
Nolan JM, Power R, Stringham J, Dennison J, Stack J, Kelly D, et al. Enrichment of macular pigment enhances contrast sensitivity in subjects free of retinal disease: central retinal enrichment supplementation trials—report 1. Invest Ophthalmol Vis Sci. 2016;57:3429–39.
Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:674–85.
Berendschot TT, van Norren D. Macular pigment shows ringlike structures. Invest Ophthalmol Vis Sci. 2006;47:709–14.
Delori FC, Goger DG, Keilhauer C, Salvetti P, Staurenghi G. Bimodal spatial distribution of macular pigment: evidence of a gender relationship. J Opt Soc Am A Opt Image Sci Vis. 2006;23:521–38.
Dietzel M, Zeimer M, Heimes B, Pauleikhoff D, Hense HW. The ringlike structure of macular pigment in age-related maculopathy: results from the Muenster Aging and Retina Study (MARS). Invest Ophthalmol Vis Sci. 2011;52:8016–24.
Sharifzadeh M, Bernstein PS, Gellermann W. Nonmydriatic fluorescence-based quantitative imaging of human macular pigment distributions. J Opt Soc Am A Opt Image Sci Vis. 2006;23:2373–87.
Sharifzadeh M, Zhao DY, Bernstein PS, Gellermann W. Resonance Raman imaging of macular pigment distributions in the human retina. J Opt Soc Am A Opt Image Sci Vis. 2008;25:947–57.
Aleman TS, Duncan JL, Bieber ML, de Castro E, Marks DA, Gardner LM, et al. Macular pigment and lutein supplementation in retinitis pigmentosa and Usher syndrome. Invest Ophthalmol Vis Sci. 2001;42:1873–81.
Liew SH, Gilbert CE, Spector TD, Mellerio J, Van Kuijk FJ, Beatty S, et al. Central retinal thickness is positively correlated with macular pigment optical density. Exp Eye Res. 2006;82:915–20.
Kanis MJ, Berendschot TT, van Norren D. Interocular agreement in melanin and macular pigment optical density. Exp Eye Res. 2007;84:934–8.
Nolan JM, Stringham JM, Beatty S, Snodderly DM. Spatial profile of macular pigment and its relationship to foveal architecture. Invest Ophthalmol Vis Sci. 2008;49:2134–42.
van der Veen RL, Ostendorf S, Hendrikse F, Berendschot TT. Macular pigment optical density relates to foveal thickness. Eur J Ophthalmol. 2009;19:836–41.
Kirby ML, Galea M, Loane E, Stack J, Beatty S, Nolan JM. Foveal anatomic associations with the secondary peak and the slope of the macular pigment spatial profile. Invest Ophthalmol Vis Sci. 2009;50:1383–91.
Wagner-Schuman M, Dubis AM, Nordgren RN, Lei Y, Odell D, Chiao H, et al. Race- and sex-related differences in retinal thickness and foveal pit morphology. Invest Ophthalmol Vis Sci. 2011;52:625–34.
Meyer zu Westrup V, Dietzel M, Pauleikhoff D, Hense HW. The association of retinal structure and macular pigment distribution. Invest Ophthalmol Vis Sci. 2014;55:1169–75.
Balaratnasingam C, Chae B, Remmer MH, Gomez E, Suzuki M, Engelbert M, et al. The spatial profile of macular pigments is related to the topological characteristics of the foveal avascular zone. Invest Ophthalmol Vis Sci. 2015;56:7859–65.
Ctori I, Huntjens B. The association between foveal morphology and macular pigment spatial distribution: an ethnicity study. PLoS ONE. 2017;12:e0169520.
Wolf-Schnurrbusch UE, Roosli N, Weyermann E, Heldner MR, Hohne K, Wolf S. Ethnic differences in macular pigment density and distribution. Invest Ophthalmol Vis Sci. 2007;48:3783–7.
Gass JD. Muller cell cone, an overlooked part of the anatomy of the fovea centralis: hypotheses concerning its role in the pathogenesis of macular hole and foveomacualr retinoschisis. Arch Ophthalmol. 1999;117:821–3.
Obana A, Sasano H, Okazaki S, Otsuki Y, Seto T, Gohto Y. Evidence of carotenoid in surgically removed lamellar hole-associated epiretinal proliferation. Invest Ophthalmol Vis Sci. 2017;58:5157–63.
Helb HM, Charbel Issa P, van der Veen RLP, Berendschot TTJM, Scholl HPN, Holz FG. Abnormal macular pigment distribution in type 2 idiopathic macular telangiectasia. Retina. 2008;28:808–16.
Zeimer MB, Padge B, Heimes B, Pauleikhoff D. Idiopathic macular telangiectasia type 2: distribution of macular pigment and functional investigations. Retina. 2010;30:586–95.
Powner MB, Gillies MC, Tretiach M, Scott A, Guymer RH, Hageman GS, et al. Perifoveal muller cell depletion in a case of macular telangiectasia type 2. Ophthalmology. 2010;117:2407–16.
Curcio CA. Antecedents of soft drusen, the specific deposits of age-related macular degeneration, in the biology of human macula. Invest Ophthalmol Vis Sci. 2018;59:AMD182–AMD194.
Obana A, Gellermann W, Gohto Y, Seto T, Sasano H, Tanito M, et al. Reliability of a two-wavelength autofluorescence technique by Heidelberg Spectralis to measure macular pigment optical density in Asian subjects. Exp Eye Res. 2018;168:100–6.
Ctori I, Huntjens B. Repeatability of foveal measurements using Spectralis optical coherence tomography segmentation software. PLoS ONE. 2015;10:e0129005.
Trieschmann M, Heimes B, Hense HW, Pauleikhoff D. Macular pigment optical density measurement in autofluorescence imaging: comparison of one- and two-wavelength methods. Graefes Arch Clin Exp Ophthalmol. 2006;244:1565–74.
Canovas R, Lima VC, Garcia P, Morini C, Prata TS, Rosen RB. Comparison between macular pigment optical density measurements using two-wavelength autofluorescence and heterochromatic flicker photometry techniques. Invest Ophthalmol Vis Sci. 2010;51:3152–6.
Creuzot-Garcher C, Koehrer P, Picot C, Aho S, Bron AM. Comparison of two methods to measure macular pigment optical density in healthy subjects. Invest Ophthalmol Vis Sci. 2014;55:2941–6.
Hammond BR Jr, Curran-Celentano J, Judd S, Fuld K, Krinsky NI, Wooten BR, et al. Sex differences in macular pigment optical density: relation to plasma carotenoid concentrations and dietary patterns. Vision Res. 1996;36:2001–122.
Broekmans WMR, Berendschot T, Klöpping-Ketelaars I, de Vries AJ, Goldbohm RA, Tijburg LBM, et al. Macular pigment density in relation to serum and adipose tissue concentrations of lutein and serum concentrations of zeaxanthin. Am J Clin Nutr. 2002;76:595–603.
Hammond BR Jr, Ciulla TA, Snodderly DM. Macular pigment density is reduced in obese subjects. Invest Ophthalmol Vis Sci. 2002;43:47–50.
Howells O, Eperjesi F, Bartlett H. Macular pigment optical density in young adults of South Asian origin. Invest Ophthalmol Vis Sci. 2013;54:2711–9.
Sasamoto Y, Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Nishida K. Effect of cataract in evaluation of macular pigment optical density by autofluorescence spectrometry. Invest Ophthalmol Vis Sci. 2011;52:927–32.
Sharifzadeh M, Obana A, Gohto Y, Seto T, Gellermann W. Autofluorescence imaging of macular pigment: influence and correction of ocular media opacities. J Biomed Opt. 2014;19:96010.
Akuffo KO, Nolan JM, Stack J, Power R, Kirwan C, Moran R, et al. The impact of cataract, and its surgical removal, on measures of macular pigment using the Heidelberg Spectralis HRA+OCT MultiColor device. Invest Ophthalmol Vis Sci. 2016;57:2552–633.
Obana A, Gohto Y, Sasano H, Gellermann W, Sharifzadeh M, Seto T, et al. Grade of cataract and its influence on measurement of macular pigment optical density using autofluorescence imaging. Invest Ophthalmol Vis Sci. 2018;59:3011–9.
Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Invest Ophthalmol Vis Sci. 2001;42:439–46.
Bernstein PS, Zhao DY, Wintch SW, Ermakov IV, McClane RW, Gellermann W. Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients. Ophthalmology. 2002;109:1780–7.
Obana A, Gohto Y, Tanito M, Okazaki S, Gellermann W, Bernstein PS, et al. Effect of age and other factors on macular pigment optical density measured with resonance Raman spectroscopy. Graefes Arch Clin Exp Ophthalmol. 2014;252:1221–8.
Delori FC, Goger DG, Hammond BR, Snodderly DM, Burns SA. Macular pigment density measured by autofluorescence spectrometry: comparison with reflectometry and heterochromatic flicker photometry. J Opt Soc Am A Opt Image Sci Vis. 2001;18:1212–30.
Berendschot TTJM, Willemse-Assink JJM, Bastiaanse M, de Jong PTVM, van Norren D. Macular pigment and melanin in age-related maculopathy in a general population. Invest Ophthalmol Vis Sci. 2002;43:1928–32.
Ciulla TA, Hammond BR Jr. Macular pigment density and aging, assessed in the normal elderly and those with cataracts and age-related macular degeneration. Am J Ophthalmol. 2004;138:582–7.
Alassane S, Binquet C, Arnould L, Fleck O, Acar N, Delcourt C, et al. Spatial distribution of macular pigment in an elderly French population: the Montrachet Study. Invest Ophthalmol Vis Sci. 2016;57:4469–75.
Kirby ML, Beatty S, Loane E, Akkali MC, Connolly EE, Stack J, et al. A central dip in the macular pigment spatial profile is associated with age and smoking. Invest Ophthalmol Vis Sci. 2010;51:6722–8.
Asefzadeh B, Cavallerano AA, Fisch BM. Racial differences in macular thickness in healthy eyes. Optom Vis Sci. 2007;84:941–5.
Duan XR, Liang YB, Friedman DS, Sun LP, Wong TY, Tao QS, et al. Normal macular thickness measurements using optical coherence tomography in healthy eyes of adult Chinese persons: the Handan Eye Study. Ophthalmology. 2010;117:1585–94.
Syrbe S, Kuhrt H, Gartner U, Habermann G, Wiedemann P, Bringmann A, et al. Muller glial cells of the primate foveola: an electron microscopical study. Exp Eye Res. 2018;167:110–7.
Amin RH, Frank RN, Kennedy A, Eliott D, Puklin JE, Abrams GW. Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 1997;38:36–47.
Behzadian MA, Wang XL, Al-Shabrawey M, Caldwell RB. Effects of hypoxia on glial cell expression of angiogenesis-regulating factors VEGF and TGF-beta. Glia. 1998;24:216–25.
Bringmann A, Pannicke T, Biedermann B, Francke M, Iandiev I, Grosche J, et al. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem Int. 2009;5:143–60.
Wang JS, Kefalov VJ. The cone-specific visual cycle. Prog Retin Eye Res. 2011;30:115–28.
Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013;61:651–78.
Franze K, Grosche J, Skatchkov SN, Schinkinger S, Foja C, Schild D, et al. Müller cells are living optical fibers in the vertebrate retina. Proc Natl Acad Sci USA. 2007;104:8287–92.
Kirschfeld K. Do Müller cells act as optical fibers in the primate retina? Invest Ophthalmol Vis Sci. 2019;60:345–8.
Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem. 2004;279:49447–54.
Li B, Vachali P, Frederick JM, Bernstein PS. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry. 2011;50:2541–9.
Acknowledgements
The authors would like to thank Jan Dechent of Heidelberg Engineering for lending us a prototype MPOD module that was installed on our Heidelberg Spectralis MultiColor platform. The authors would also like to thank Chika Wada and Ryoki Ashino for their assistance with OCT measurement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
A. Obana, None; Y. Gohto, None; H. Sasano, None; W. Gellermann, None; M. Sharifzadeh, None; T. Seto, None; P. S. Bernstein, None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Akira Obana
About this article
Cite this article
Obana, A., Gohto, Y., Sasano, H. et al. Spatial distribution of macular pigment estimated by autofluorescence imaging in elderly Japanese individuals. Jpn J Ophthalmol 64, 160–170 (2020). https://doi.org/10.1007/s10384-020-00716-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-020-00716-5